filmov
tv
Effective Nuclear Charge - Chemistry Tutorial
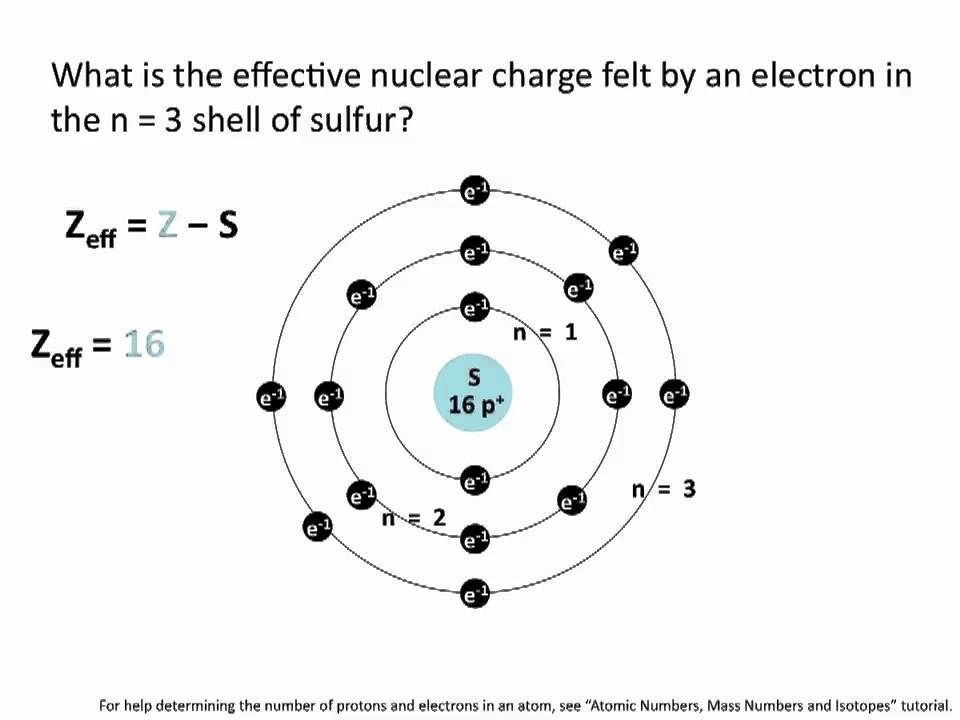
Показать описание
This chemistry tutorial covers how to calculate the average effective nuclear charge felt by an electron in any shell in at atom.
How To Calculate The Effective Nuclear Charge of an Electron
Effective Nuclear Charge - Chemistry Tutorial
Nuclear Charge, Shielding Effect & Effective Nuclear Charge (Singapore A Level H2 Chemistry)
What is Effective Nuclear Force?
Effective Nuclear Charge, Shielding effect, & Periodic Properties Tutorial; Crash Chemistry Acad...
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
How To Use Slater's Rule to Estimate The Effective Nuclear Charge
Shielding Effect and Effective Nuclear Charge
Zeff Nuclear Charge CSIR NET Chemistry | Atomic Structure & Nuclear Chemistry CSIR NET 2024 | IF...
Effective Nuclear Charge
calculating effective nuclear charge
Trick for Slater's Rule, calculation of screening constant and effective nuclear charge.
Effective Nuclear Charge :Definition, Examples ,Trend ,Formula & Calculation | Chemistry
Shielding Effect in the Periodic Table | Chemistry
Atomic Radius - Basic Introduction - Periodic Table Trends, Chemistry
Effective nuclear charge - Real Chemistry
Chemistry Part 23: Effective Nuclear Charge
11C02 - Atomic Structure - Effective Nuclear Charge & Screening Effect - Ashwin Sir
Effective nuclear charge (Zeff)|Chemistry
Difference between Nuclear Charge and Effective Nuclear Charge | Periodic Trends Chemistry
Effective Nuclear Charge
What is effective nuclear charge ?
7.1b Slater's Rules | General Chemistry
8.Chemistry | Periodic classification of elements | Effective nuclear charge 1
Комментарии
 0:07:14
0:07:14
 0:04:47
0:04:47
 0:01:47
0:01:47
 0:07:17
0:07:17
 0:17:27
0:17:27
 0:07:53
0:07:53
 0:12:30
0:12:30
 0:03:05
0:03:05
 1:11:42
1:11:42
 0:02:58
0:02:58
 0:04:05
0:04:05
 0:09:31
0:09:31
 0:11:15
0:11:15
 0:04:58
0:04:58
 0:14:04
0:14:04
 0:10:50
0:10:50
 0:05:25
0:05:25
 0:07:20
0:07:20
 0:04:23
0:04:23
 0:05:07
0:05:07
 0:05:28
0:05:28
 0:03:52
0:03:52
 0:15:17
0:15:17
 0:09:12
0:09:12