filmov
tv
The Mole and the Avogadro's Constant | A-level Chemistry | OCR, AQA, Edexcel

Показать описание
The Mole and the Avogadro Constant in a Snap!
SnapRevise is the UK’s leading A-level and GCSE revision & exam preparation resource offering comprehensive video courses created by A* tutors. Our courses are designed around the OCR, AQA, SNAB, Edexcel B, WJEC, CIE and IAL exam boards, concisely covering all the important concepts required by each specification. In addition to all the content videos, our courses include hundreds of exam question videos, where we show you how to tackle questions and walk you through step by step how to score full marks.
Sign up today and together, let’s make A-level Chemistry a walk in the park!
The key points covered in this video include:
1. What is the Mole?
2. The Mole
3. Molar Mass
4. Equations
What is the Mole?
Difficult to count the number of atoms accurately. Tiny size! In order to quantify the amount of a substance scientists use a measure called the mole. A mole is: The amount od a substance that contains the same number of atoms or particles as 12g of carbon-12.
The Mole
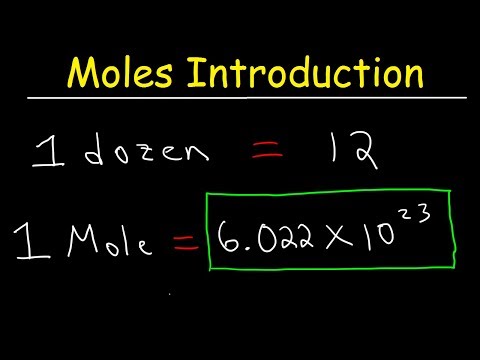
The value of the mole is: 6.02 x 10^23. Also known as Avogadro’s Constant. Huge number!
Molar Mass
Mass of one mole of a substance. Takes the same value as Atomic/Molecular/Formula Mass. Units: g mol^-1.
Equations
n - Number of moles. Units: mol. m - Mass. Units: g. M - Molar Mass. Units: g mol^-1.
SnapRevise is the UK’s leading A-level and GCSE revision & exam preparation resource offering comprehensive video courses created by A* tutors. Our courses are designed around the OCR, AQA, SNAB, Edexcel B, WJEC, CIE and IAL exam boards, concisely covering all the important concepts required by each specification. In addition to all the content videos, our courses include hundreds of exam question videos, where we show you how to tackle questions and walk you through step by step how to score full marks.
Sign up today and together, let’s make A-level Chemistry a walk in the park!
The key points covered in this video include:
1. What is the Mole?
2. The Mole
3. Molar Mass
4. Equations
What is the Mole?
Difficult to count the number of atoms accurately. Tiny size! In order to quantify the amount of a substance scientists use a measure called the mole. A mole is: The amount od a substance that contains the same number of atoms or particles as 12g of carbon-12.
The Mole
The value of the mole is: 6.02 x 10^23. Also known as Avogadro’s Constant. Huge number!
Molar Mass
Mass of one mole of a substance. Takes the same value as Atomic/Molecular/Formula Mass. Units: g mol^-1.
Equations
n - Number of moles. Units: mol. m - Mass. Units: g. M - Molar Mass. Units: g mol^-1.
Комментарии
 0:06:06
0:06:06
 0:04:33
0:04:33
 0:13:37
0:13:37
 0:08:57
0:08:57
 0:04:02
0:04:02
 0:07:14
0:07:14
 0:10:28
0:10:28
 0:03:41
0:03:41
 0:04:29
0:04:29
 0:07:02
0:07:02
 0:05:58
0:05:58
 0:04:02
0:04:02
 0:05:16
0:05:16
 0:09:57
0:09:57
 0:10:50
0:10:50
 0:09:49
0:09:49
 0:04:22
0:04:22
 0:06:00
0:06:00
 0:39:29
0:39:29
 0:23:44
0:23:44
 0:02:58
0:02:58
 0:01:07
0:01:07
 0:14:04
0:14:04
 0:20:54
0:20:54