filmov
tv
The mole and Avogadro's number | Moles and molar mass | High school chemistry | Khan Academy

Показать описание
Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now!
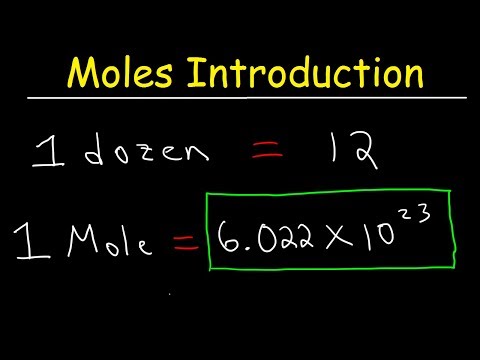
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro's number or Avogadro's constant. The concept of the mole can be used to convert between mass and number of particles.
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro's number or Avogadro's constant. The concept of the mole can be used to convert between mass and number of particles.
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
The Mole: Avogadro's Number and Stoichiometry
The mole and Avogadro's number | Atomic structure and properties | AP Chemistry | Khan Academy
Mole and Avogadro's Number | Chemistry
What Is Avogadro's Number - The Mole | Chemical Calculations | Chemistry | FuseSchool
How big is a mole? (Not the animal, the other one.) - Daniel Dulek
The Big Idea Behind Avogadro's Number (That Most People Miss)
An Actually Good Explanation of Moles
The MOLE & Avogadro's Number (Chemistry)
GCSE Chemistry - The Mole (Higher Tier) #25
The mole and Avogadro's number | Moles and molar mass | High school chemistry | Khan Academy
An Easy Way to Understanding the Mole and Avogadros Number Part 14
Using Avogadro’s Number | How to Pass Chemistry
Concept of Mole | Avogadro's Number | Atoms and Molecules | Don't Memorise
The Mole
Chemistry Lesson: The Mole (Avogadro's Number)
Introduction to Moles
Introduction to Moles
What is a mole in terms of Avogadro's constant
Complete History of the Avogadro Number
1-1 The Mole & Avogadro's Number
Mole and Avogadro s Number
Avogadro's Number (Mole) - Numberphile
Converting Between Moles, Atoms, and Molecules
Calculating Mole Grade 10 | Part 1
Комментарии
 0:06:06
0:06:06
 0:08:57
0:08:57
 0:07:14
0:07:14
 0:04:02
0:04:02
 0:04:33
0:04:33
 0:07:29
0:07:29
 0:13:37
0:13:37
 0:10:28
0:10:28
 0:04:29
0:04:29
 0:03:41
0:03:41
 0:05:58
0:05:58
 0:02:47
0:02:47
 0:06:00
0:06:00
 0:07:02
0:07:02
 0:09:49
0:09:49
 0:10:50
0:10:50
 0:05:16
0:05:16
 0:03:01
0:03:01
 0:34:55
0:34:55
 0:04:19
0:04:19
 0:14:27
0:14:27
 0:09:57
0:09:57
 0:14:00
0:14:00
 0:11:46
0:11:46