filmov
tv
What is Chlor Alkali Process | chlorine preparation | Hindi tutorial | class 10 | #shorts
Показать описание
What is Chlor Alkali Process | chlorine preparation | Hindi tutorial | class 10 | #shorts
#ytshort
#class10acidbase
#science
#tutorialyoutube
#tutorials
#tutorialscience
#howtoconvert
#howto
#process
#class10sciencechemicalreactionsandequations
What is chlor-alkali process in class 10th?
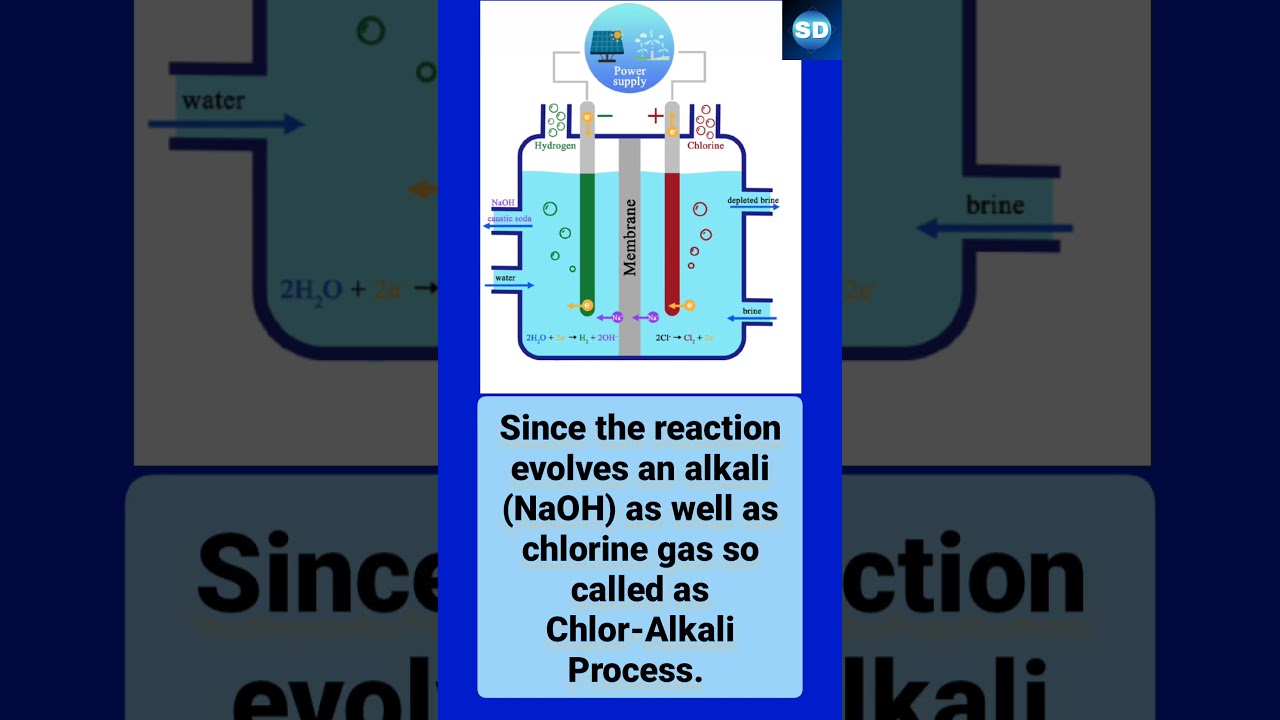
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process. This process is taken place by electrolysis of aqueous chloride.
What are the three products of the chlor-alkali process?
When the concentrated Sodium chloride undergoes electrolysis, then three products are formed. The product formed are: Sodium hydroxide ( ), Chlorine gas ( C l 2 ), and Hydrogen gas ( ).
कक्षा 10 वीं में क्लोर-क्षार प्रक्रिया क्या है?
जब सोडियम क्लोराइड (जिसे ब्राइन कहा जाता है) के जलीय घोल से बिजली प्रवाहित की जाती है, तो यह विघटित होकर सोडियम हाइड्रॉक्साइड बन जाता है। इस प्रक्रिया को क्लोर-क्षार प्रक्रिया कहा जाता है। यह प्रक्रिया जलीय क्लोराइड के इलेक्ट्रोलिसिस द्वारा होती है।
What is alkali class 10?
Alkali is defined as an ionic or basic salt of an alkaline earth metal or alkali metal. It is a base that can dissolve in water.
What is brine formula?
Overall process: 2 NaCl + 2 H 2O → Cl 2 + H 2 + 2 NaOH.
What is brine class 10?
Brine is a saturated or strongly concentrated solution of water and salt especially sodium chloride.
How is NaOH produced from NaCl?
Commercially, NaOH is manufactured by the electrolysis of brine (NaCl solution).
What is the equation for the chlor-alkali process?
Is NaOH acidic or basic?
base
NaOH (sodium hydroxide) is a base. According to definitions, anything that contains hydroxide-ion and releases it in water is considered a base.
#ytshort
#class10acidbase
#science
#tutorialyoutube
#tutorials
#tutorialscience
#howtoconvert
#howto
#process
#class10sciencechemicalreactionsandequations
What is chlor-alkali process in class 10th?
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process. This process is taken place by electrolysis of aqueous chloride.
What are the three products of the chlor-alkali process?
When the concentrated Sodium chloride undergoes electrolysis, then three products are formed. The product formed are: Sodium hydroxide ( ), Chlorine gas ( C l 2 ), and Hydrogen gas ( ).
कक्षा 10 वीं में क्लोर-क्षार प्रक्रिया क्या है?
जब सोडियम क्लोराइड (जिसे ब्राइन कहा जाता है) के जलीय घोल से बिजली प्रवाहित की जाती है, तो यह विघटित होकर सोडियम हाइड्रॉक्साइड बन जाता है। इस प्रक्रिया को क्लोर-क्षार प्रक्रिया कहा जाता है। यह प्रक्रिया जलीय क्लोराइड के इलेक्ट्रोलिसिस द्वारा होती है।
What is alkali class 10?
Alkali is defined as an ionic or basic salt of an alkaline earth metal or alkali metal. It is a base that can dissolve in water.
What is brine formula?
Overall process: 2 NaCl + 2 H 2O → Cl 2 + H 2 + 2 NaOH.
What is brine class 10?
Brine is a saturated or strongly concentrated solution of water and salt especially sodium chloride.
How is NaOH produced from NaCl?
Commercially, NaOH is manufactured by the electrolysis of brine (NaCl solution).
What is the equation for the chlor-alkali process?
Is NaOH acidic or basic?
base
NaOH (sodium hydroxide) is a base. According to definitions, anything that contains hydroxide-ion and releases it in water is considered a base.
 0:02:31
0:02:31
 0:00:45
0:00:45
 0:33:35
0:33:35
 0:04:58
0:04:58
 0:05:23
0:05:23
 0:00:46
0:00:46
 0:00:50
0:00:50
 0:00:51
0:00:51
 0:07:10
0:07:10
 0:00:50
0:00:50
 0:03:30
0:03:30
 0:05:42
0:05:42
 0:05:46
0:05:46
 0:11:50
0:11:50
 0:23:39
0:23:39
 0:05:34
0:05:34
 0:19:54
0:19:54
 0:01:15
0:01:15
 0:00:06
0:00:06
 0:01:28
0:01:28
 0:00:47
0:00:47
 0:06:57
0:06:57
 0:10:04
0:10:04
 0:03:32
0:03:32