filmov
tv
Chemical Effects of Electric Current Class 8 Science - Electroplating
Показать описание
Chemical Effects of Electric Current Class 8 Science - Electroplating
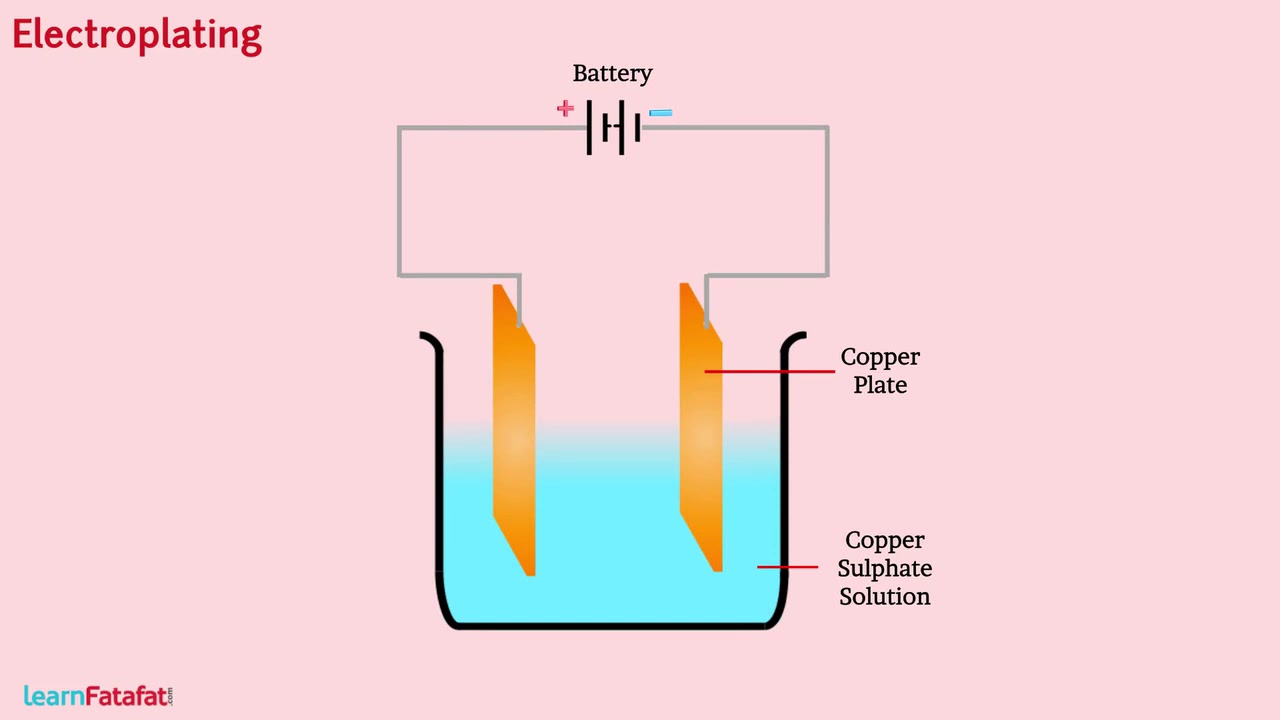
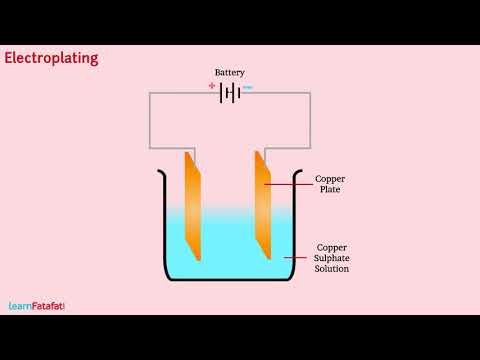
A process of depositing a layer of any desired metal on another material by means of electricity is called electroplating. It is one of the most common applications of chemical effects of electric current.
When electric current is passed through the copper sulphate solution there is dissociation (Separation of ions) of copper sulphate. Dissociation signifies separation of positively charged copper ion from negatively charged sulphate ion. Being positively charged the copper ions get attracted towards negatively charged copper plate, where the ion receives electrons and get neutralised. During same time electrons get pulled by positive terminal of battery from the copper plate connected to positive terminal, as a result a neutral copper atom get converted to ion. The ion move in the solution. The process continues as long as current flows through the solution making a coat of copper on the plate connected to negative terminal.
Such a process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
A process of depositing a layer of any desired metal on another material by means of electricity is called electroplating. It is one of the most common applications of chemical effects of electric current.
When electric current is passed through the copper sulphate solution there is dissociation (Separation of ions) of copper sulphate. Dissociation signifies separation of positively charged copper ion from negatively charged sulphate ion. Being positively charged the copper ions get attracted towards negatively charged copper plate, where the ion receives electrons and get neutralised. During same time electrons get pulled by positive terminal of battery from the copper plate connected to positive terminal, as a result a neutral copper atom get converted to ion. The ion move in the solution. The process continues as long as current flows through the solution making a coat of copper on the plate connected to negative terminal.
Such a process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
Комментарии
 0:02:45
0:02:45
 0:06:24
0:06:24
 1:09:30
1:09:30
 0:09:39
0:09:39
 0:39:49
0:39:49
 0:18:25
0:18:25
 0:12:28
0:12:28
 0:04:55
0:04:55
 0:29:15
0:29:15
 0:57:03
0:57:03
 0:06:43
0:06:43
 0:09:57
0:09:57
 0:32:28
0:32:28
 0:17:10
0:17:10
 0:52:02
0:52:02
 0:17:34
0:17:34
 0:06:27
0:06:27
 0:02:29
0:02:29
 0:18:40
0:18:40
 7:14:48
7:14:48
 0:25:15
0:25:15
 0:46:13
0:46:13
 0:34:54
0:34:54
 0:04:14
0:04:14