filmov
tv
Chapter 17 – Reactions of Carbonyl Compounds: Part 2 of 4
Показать описание
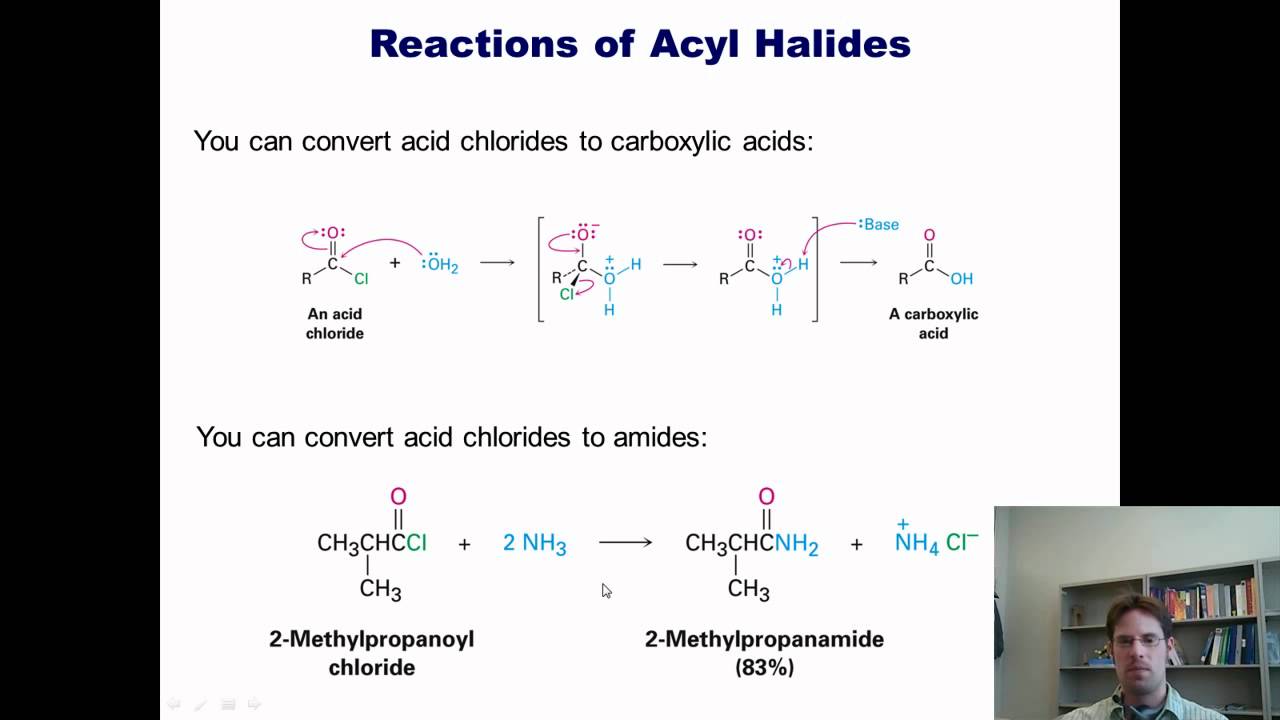
In this video I'll begin teaching you about how carbonyl compounds react. I begin by comparing their mechanism to a man getting hit in the groin with a football. I then teach specific reaction examples, which include reactions of acyl (acid) chlorides, acid anhydrides, esters, carboxylic acids, and amides. --Dr. Mike Christiansen from Utah State University
Chapter 17 – Reactions of Carbonyl Compounds: Part 1 of 4
Chapter 17: Reactions of Aromatic Compounds | Organic Chemistry by L.G Wade Jr.
Chapter 17 – Reactions of Carbonyl Compounds: Part 1 of 7
John 17:1–26 • The Prayer of our High Priest
Chapter 17 – Reactions of Carbonyl Compounds: Part 3 of 4
Chapter 17 – Reactions of Carbonyl Compounds: Part 2 of 4
Chapter 17 – Reactions of Carbonyl Compounds: Part 4 of 7
Chapter 17 – Reactions of Carbonyl Compounds: Part 3 of 7
A satisfying chemical reaction
Ch#17 |Lec#5 | substitution Versus Elimination Reactions Of Alkyl Halides + Factors,
Ch#17 |Lec#8 | Basicity Of Amines +Reactions Of Amines, diazonium salt Class12
Organic Chapter 17: Reactions at the Alpha Carbon Video 1 of 4
Ch#17 |Lec#3 | SN1 and SN2 Reactions & mechanism, Nucleophilic substitution Reactions
Chapter 17 – Reactions of Carbonyl Compounds: Part 6 of 7
Chapter 17 – Reactions of Carbonyl Compounds: Part 5 of 7
Ch#17 |Lec#4 | E1 and E2 Reactions and mechanism, Elimination Reactions and types
Ch#17||Lec#6 |Grignard reagent, Organo metallic Compounds, Reactions & reactivity, preparation
Grignard Reagent Reaction Mechanism
Chapter 17 – Reactions of Carbonyl Compounds: Part 7 of 7
Organic Chemistry 2: Chapter 17 - Aromatic Compounds (Part 1/1)
BIO 205 - Chapters 17 & 18 - Innate Nonspecific Host Defenses and Adaptive Specific Host Defense...
Chapter 17 Practice Problems
Chapter 17 – Reactions of Carbonyl Compounds: Part 2 of 7
Chapters 17 and 18 Lecture — Chemical Reactions
Комментарии
 0:14:40
0:14:40
 0:40:12
0:40:12
 0:08:10
0:08:10
 0:47:32
0:47:32
 0:19:48
0:19:48
 0:27:27
0:27:27
 0:08:45
0:08:45
 0:08:51
0:08:51
 0:00:19
0:00:19
 0:19:24
0:19:24
 0:30:00
0:30:00
 0:14:39
0:14:39
 0:28:42
0:28:42
 0:09:58
0:09:58
 0:09:49
0:09:49
 0:24:03
0:24:03
 0:31:44
0:31:44
 0:12:50
0:12:50
 0:09:46
0:09:46
 0:37:30
0:37:30
 1:01:45
1:01:45
 0:44:45
0:44:45
 0:06:18
0:06:18
 1:13:18
1:13:18