filmov
tv
Why Are pH Values Only In A Range Of 0-14?
Показать описание
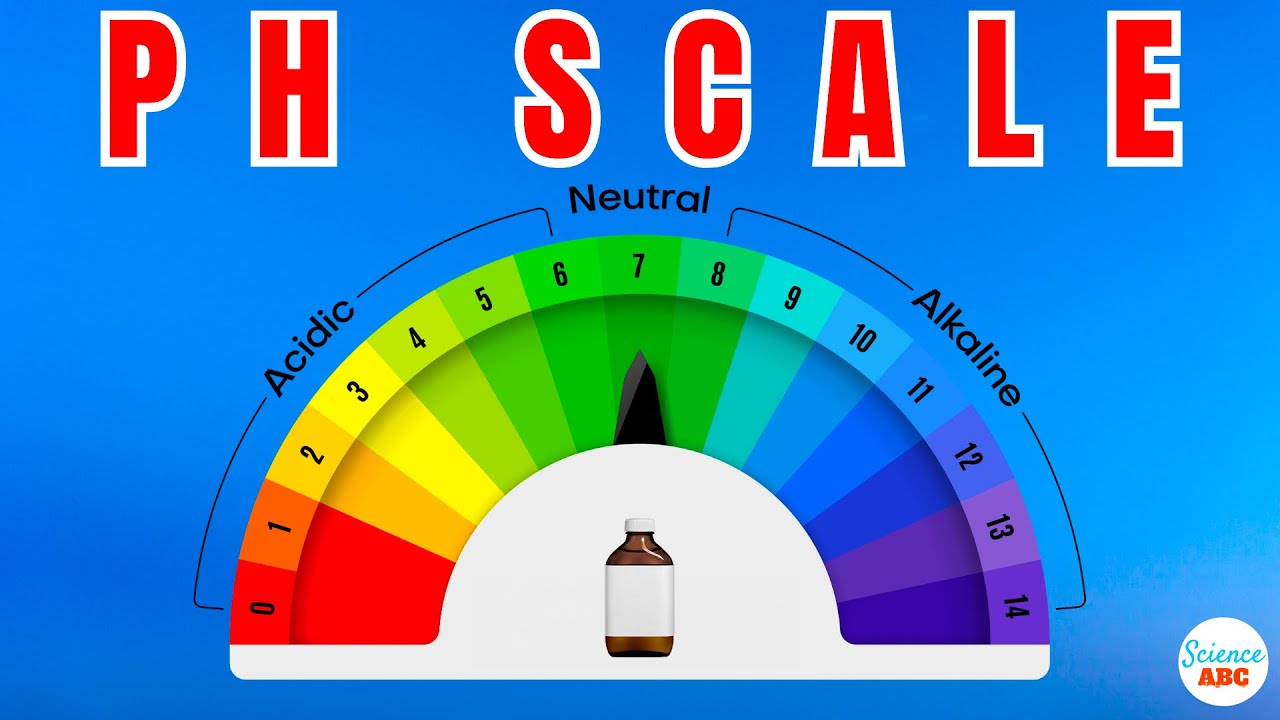
The pH scale is used to determine whether a substance is acidic or basic, and to calculate how strong a chemical it is. A pH value is a number that ranges from 1 to 14 for most common chemicals, with seven being the middle or neutral point. Values below 7 are indicators of acidity, which increases as the number decreases, while values above 7 indicate alkalinity, which increases as the value increases. An important distinction to understand is that the pH scale is a logarithmic scale.
On this scale, a substance with a pH of 3 is ten times more acidic than a substance with a pH of 4, and 100 times more acidic than a substance with a pH of 5. Similarly, a substance with a pH of 9 is ten times more alkaline than a substance with a pH of 8, and 1000 times more alkaline than a substance with a pH of 6.
Theoretically speaking, the pH scale should actually range from negative infinity to positive infinity. This claim is according to its definition, which states that the pH of a substance is the value defined by the negative logarithm of the hydrogen ion concentration. However, in reality, most solutions you would find in a standard laboratory have a pH value between 0-14.
Stock Music Source:
––––––––––––––––––––––––––––––
Track: Outer Space — SOMM [Audio Library Release]
Music provided by Audio Library Plus
––––––––––––––––––––––––––––––
#phscale #phvalue #chemistry
References:
SUBSCRIBE to get more such science videos!
Follow us on Twitter!
Follow us on Facebook!
Follow our Website!
On this scale, a substance with a pH of 3 is ten times more acidic than a substance with a pH of 4, and 100 times more acidic than a substance with a pH of 5. Similarly, a substance with a pH of 9 is ten times more alkaline than a substance with a pH of 8, and 1000 times more alkaline than a substance with a pH of 6.
Theoretically speaking, the pH scale should actually range from negative infinity to positive infinity. This claim is according to its definition, which states that the pH of a substance is the value defined by the negative logarithm of the hydrogen ion concentration. However, in reality, most solutions you would find in a standard laboratory have a pH value between 0-14.
Stock Music Source:
––––––––––––––––––––––––––––––
Track: Outer Space — SOMM [Audio Library Release]
Music provided by Audio Library Plus
––––––––––––––––––––––––––––––
#phscale #phvalue #chemistry
References:
SUBSCRIBE to get more such science videos!
Follow us on Twitter!
Follow us on Facebook!
Follow our Website!
Комментарии
 0:03:38
0:03:38
 0:04:00
0:04:00
 0:08:36
0:08:36
 0:03:11
0:03:11
 0:00:41
0:00:41
 0:00:58
0:00:58
 0:11:23
0:11:23
 0:08:17
0:08:17
 0:02:14
0:02:14
 0:13:50
0:13:50
 0:04:37
0:04:37
 0:09:38
0:09:38
 0:07:46
0:07:46
 0:16:21
0:16:21
 0:07:06
0:07:06
 0:05:14
0:05:14
 0:05:12
0:05:12
 0:03:49
0:03:49
 0:02:17
0:02:17
 0:08:31
0:08:31
 0:08:49
0:08:49
 0:04:05
0:04:05
 0:00:16
0:00:16
 0:03:30
0:03:30