filmov
tv
8.2 Hydrohalogenation of Alkenes | Organic Chemistry

Показать описание
Chad breaks down the hydrohalogenation of alkenes in which HCl, HBr, or HI is added across an alkene. Chad shows the step-by-step reaction mechanism for the addition of HBr to propene, explaining why it results in Markovnikov addition, has no stereoselectivity, and is subject to carbocation rearrangements. Chad then shows how the addition of HBr with peroxide (HBr/ROOR) proceeds through a radical mechanism resulting in anti-Markovnikov addition of HBr.
00:00 Lesson Introduction
01:08 Introduction to Hydrohalogenation
02:44 Hydrohalogenation of Alkenes Mechanism
05:09 Hydrohalogenation of Alkenes with peroxide (Addition of HBr / ROOR)
00:00 Lesson Introduction
01:08 Introduction to Hydrohalogenation
02:44 Hydrohalogenation of Alkenes Mechanism
05:09 Hydrohalogenation of Alkenes with peroxide (Addition of HBr / ROOR)
GCSE Chemistry - Addition Reactions of Alkenes #55
Reactions of Alkenes | Addition of hydrogen halide | ch#8 | 12th class chemistry
Alkene Addition Reactions: Crash Course Organic Chemistry #16
Ch#16|Lec#8|Reactions Of Alkenes, Hydrogenation, hydro Halogenation, hydration, Halogenation,
8 - Reactions of Alkenes
Reactions of Alkenes | Hydrogenation | ch#8 | 12th class chemistry
Chem12A Chapter 8 Lecture 2 Hydration of Alkenes part 1
Hydrohalogenation of alkenes
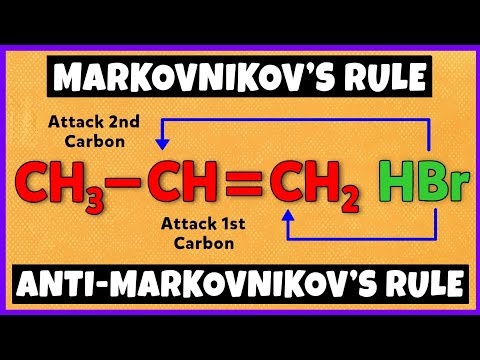
Markovnikov's Rule | Anti-Markovnikov's Rule | Mechanism
Alkenes: Addition reactions: Hydration & Hydrogen Halides | Lesson 8
Markovnikov's rule: hydrohalogenation of alkenes
NMDCAT | Chemistry of Hydrocarbons | Unit 14 | Lecture No.2 | Prof.Wajid Ali Kamboh | WAK Entry Test
Fsc Chemistry book 2, Ch 8 - Chemical Reactions of Alkenes - 12th Class Chemistry
Alkene Reactions #1 - Narrated Answer Key
Reaction of Alkene with Hypohalous acid || Reactions of Alkenes || 12th class chemistry || ch.no.8
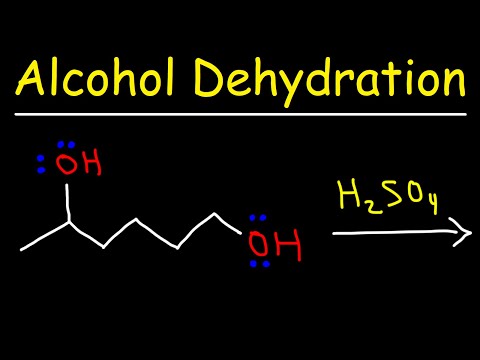
Alcohol Dehydration Reaction Mechanism With H2SO4
Organic Chemistry 1: Chapter 8 - Reactions of Alkenes (Part 1/1)
Methods of Preparation of Alkene | Dehydrohalogenation of alkyl halide | ch#8 | 12th class chemistry
Ozonolysis of Alkenes | Trick of Ozonolysis of Alkenes
Organic Mechanism : Electrophilic Addition of Alkenes : Part 2 : Hydrohalogenation
lec#8 Hydrohalogenation of alkenes ||Reactions Of Alkenes || #markownnikoves rule
Dehydration of Alcohols (Elimination, Forms Alkenes)
Organic Chemistry I CHEM-2423 Ch 10 Alkenes Part 2
Chem 12A Chapter 8 Lecture 3 Hydration of Alkenes part 2
Комментарии
 0:05:45
0:05:45
 0:24:35
0:24:35
 0:12:53
0:12:53
 0:36:18
0:36:18
 1:21:48
1:21:48
 0:17:29
0:17:29
 0:21:42
0:21:42
 0:06:54
0:06:54
 0:12:48
0:12:48
 0:07:43
0:07:43
 0:31:33
0:31:33
 1:08:44
1:08:44
 0:23:28
0:23:28
 0:19:56
0:19:56
 0:09:26
0:09:26
 0:16:59
0:16:59
 0:43:54
0:43:54
 0:08:29
0:08:29
 0:06:55
0:06:55
 0:02:36
0:02:36
 0:24:06
0:24:06
 0:11:18
0:11:18
 0:54:51
0:54:51
 0:13:17
0:13:17