filmov
tv
CHAPERONES AND MISFOLDED PROTEINS
Показать описание
In order to become a useful protein, the polypeptide produced by a ribosome during translation must be folded into a unique 3-dimentional configuration and must bind any co-factors required for its activity. It might also need to be modified appropriated by protein-modifying enzymes, such as protein kinases, or to correctly join other protein subunits.
Protein structures are evolved to fold rapidly into their final shapes, and some proteins begin the folding process as the N-terminus emerges from the ribosome. Typically, this results in a flexible state termed a “molten globule”, which contains secondary folding features – alpha helices and beta pleated sheets. While the molten globule stage is usually complete within seconds, it is merely a starting point for the then slow process of side-chain adjustments until the correct tertiary structure is achieved. This slower process usually takes several minutes.
Misfolded, or aberrant, proteins are not only a waste of energy for a cell, but they are also dangerous. These aberrant proteins typically have exposed hydrophobic regions, which cause them to clump together into aggregates, which accumulate and can cause severe diseases.
The cell has two lines of defense against the formation of these dangerous misfolded proteins – molecular chaperones, and the proteasome. Molecular chaperones help proteins fold correctly, while the proteasome destroys misfolded proteins through proteolysis. The molecular chaperones and proteasomes compete over these proteins. The longer a protein remains misfolded, either because chaperones haven’t reached it, or because they haven’t succeeded in fixing it, the more likely it is that a proteasome will degrade it.
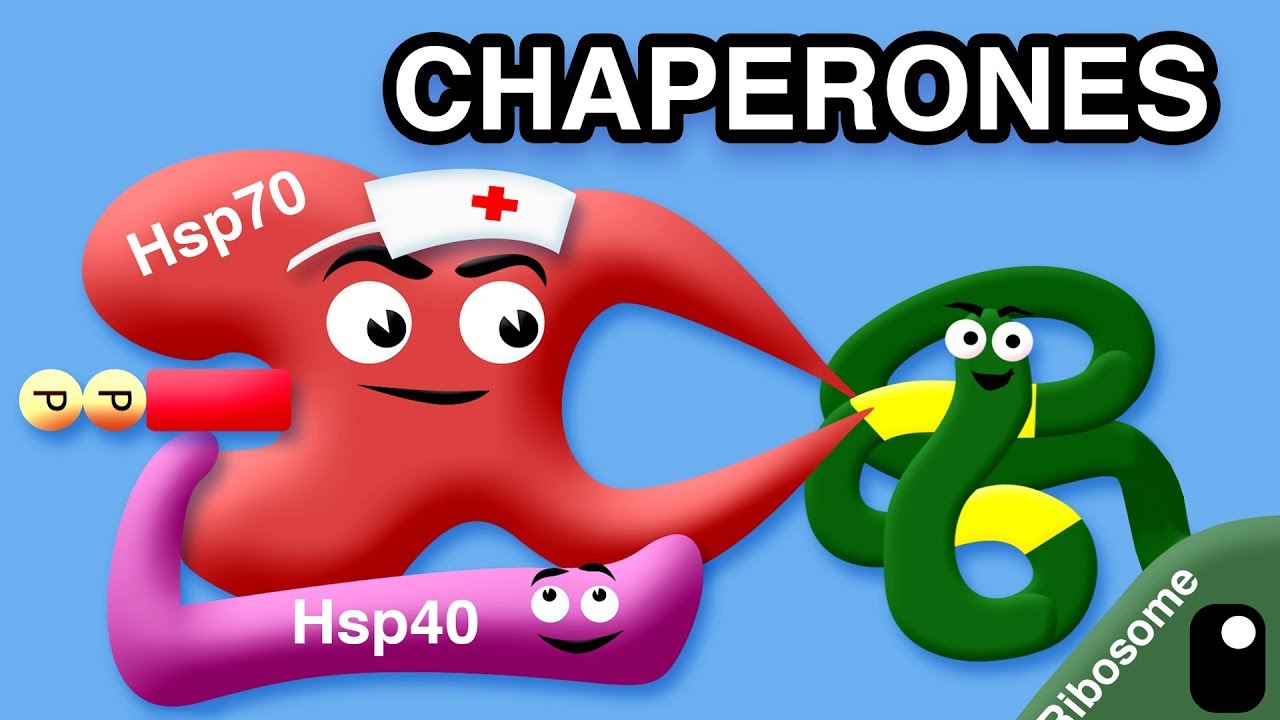
Let’s discuss chaperones first. Chaperones work by binding to exposed hydrophobic patches on misfolded or incompletely folded proteins and hydrolyzing ATP. Some of the energy expended by chaperones is used to perform mechanical work, but much more is used to ensure the accuracy of protein folding . The proteins that chaperones help fold properly are called “client proteins”.
There are several families of molecular chaperones in eukaryotes, which function in different organelles. The ones we will be discussing are Hsp60 and Hsp70, each of which has a set of associated proteins. Hsp stands for heat-shock proteins, because lots of them are produced when a cell is exposed to elevated temperatures, responding to increased amounts of misfolded proteins.
Hsp70 acts early on in the process of protein folding, binding to polypeptide chains emerging from ribosomes where there is a chain of 7 hydrophobic amino acids. Hsp70 is aided by Hsp40s, and many cycles of ATP hydrolysis are generally required to fold a single polypeptide chain correctly.
Hsp60 acts on fully synthesized proteins, forming a large barrel that serves to isolate the protein and provide better conditions for folding. Hsp60, which is a type of molecular chaperone called a chaperonin, initially captures proteins along the rim of one of its barrels. Then, the binding of ATP and a protein cap increases the diameter of the barrel and partly unfolds the protein. Inside the barrel, the protein has a chance to fold in a more favourable environment. 15 seconds after ATP binds to the Hsp60, it hydrolyzes, weakening the complex. The binding of another ATP molecule makes the protein leave. If the protein is still not folded correctly, it can repeat the cycle.
Proteasomes are so important, that they constitute almost 1% of cell proteins. They are dispersed throughout the nucleus and cytosol. If a protein folds correctly and quickly, then none, or only a small bit of it gets degraded. A slowly-folding protein is at risk for a longer time, and more of it might get destroyed before the remaining amino acids attain the correctly folded state. I will discuss the destruction of proteins in detail in the next video.
Protein structures are evolved to fold rapidly into their final shapes, and some proteins begin the folding process as the N-terminus emerges from the ribosome. Typically, this results in a flexible state termed a “molten globule”, which contains secondary folding features – alpha helices and beta pleated sheets. While the molten globule stage is usually complete within seconds, it is merely a starting point for the then slow process of side-chain adjustments until the correct tertiary structure is achieved. This slower process usually takes several minutes.
Misfolded, or aberrant, proteins are not only a waste of energy for a cell, but they are also dangerous. These aberrant proteins typically have exposed hydrophobic regions, which cause them to clump together into aggregates, which accumulate and can cause severe diseases.
The cell has two lines of defense against the formation of these dangerous misfolded proteins – molecular chaperones, and the proteasome. Molecular chaperones help proteins fold correctly, while the proteasome destroys misfolded proteins through proteolysis. The molecular chaperones and proteasomes compete over these proteins. The longer a protein remains misfolded, either because chaperones haven’t reached it, or because they haven’t succeeded in fixing it, the more likely it is that a proteasome will degrade it.
Let’s discuss chaperones first. Chaperones work by binding to exposed hydrophobic patches on misfolded or incompletely folded proteins and hydrolyzing ATP. Some of the energy expended by chaperones is used to perform mechanical work, but much more is used to ensure the accuracy of protein folding . The proteins that chaperones help fold properly are called “client proteins”.
There are several families of molecular chaperones in eukaryotes, which function in different organelles. The ones we will be discussing are Hsp60 and Hsp70, each of which has a set of associated proteins. Hsp stands for heat-shock proteins, because lots of them are produced when a cell is exposed to elevated temperatures, responding to increased amounts of misfolded proteins.
Hsp70 acts early on in the process of protein folding, binding to polypeptide chains emerging from ribosomes where there is a chain of 7 hydrophobic amino acids. Hsp70 is aided by Hsp40s, and many cycles of ATP hydrolysis are generally required to fold a single polypeptide chain correctly.
Hsp60 acts on fully synthesized proteins, forming a large barrel that serves to isolate the protein and provide better conditions for folding. Hsp60, which is a type of molecular chaperone called a chaperonin, initially captures proteins along the rim of one of its barrels. Then, the binding of ATP and a protein cap increases the diameter of the barrel and partly unfolds the protein. Inside the barrel, the protein has a chance to fold in a more favourable environment. 15 seconds after ATP binds to the Hsp60, it hydrolyzes, weakening the complex. The binding of another ATP molecule makes the protein leave. If the protein is still not folded correctly, it can repeat the cycle.
Proteasomes are so important, that they constitute almost 1% of cell proteins. They are dispersed throughout the nucleus and cytosol. If a protein folds correctly and quickly, then none, or only a small bit of it gets degraded. A slowly-folding protein is at risk for a longer time, and more of it might get destroyed before the remaining amino acids attain the correctly folded state. I will discuss the destruction of proteins in detail in the next video.
Комментарии
 0:04:11
0:04:11
 0:07:46
0:07:46
 0:03:06
0:03:06
 0:00:10
0:00:10
 0:02:10
0:02:10
 0:03:35
0:03:35
 0:02:14
0:02:14
 0:05:58
0:05:58
 0:03:57
0:03:57
 0:10:02
0:10:02
 0:01:22
0:01:22
 0:38:10
0:38:10
 0:06:19
0:06:19
 0:01:38
0:01:38
 0:23:11
0:23:11
 0:07:48
0:07:48
 0:00:49
0:00:49
 0:00:39
0:00:39
 0:14:45
0:14:45
 0:16:38
0:16:38
 0:03:31
0:03:31
 0:00:17
0:00:17
 0:07:21
0:07:21
 0:37:12
0:37:12