filmov
tv
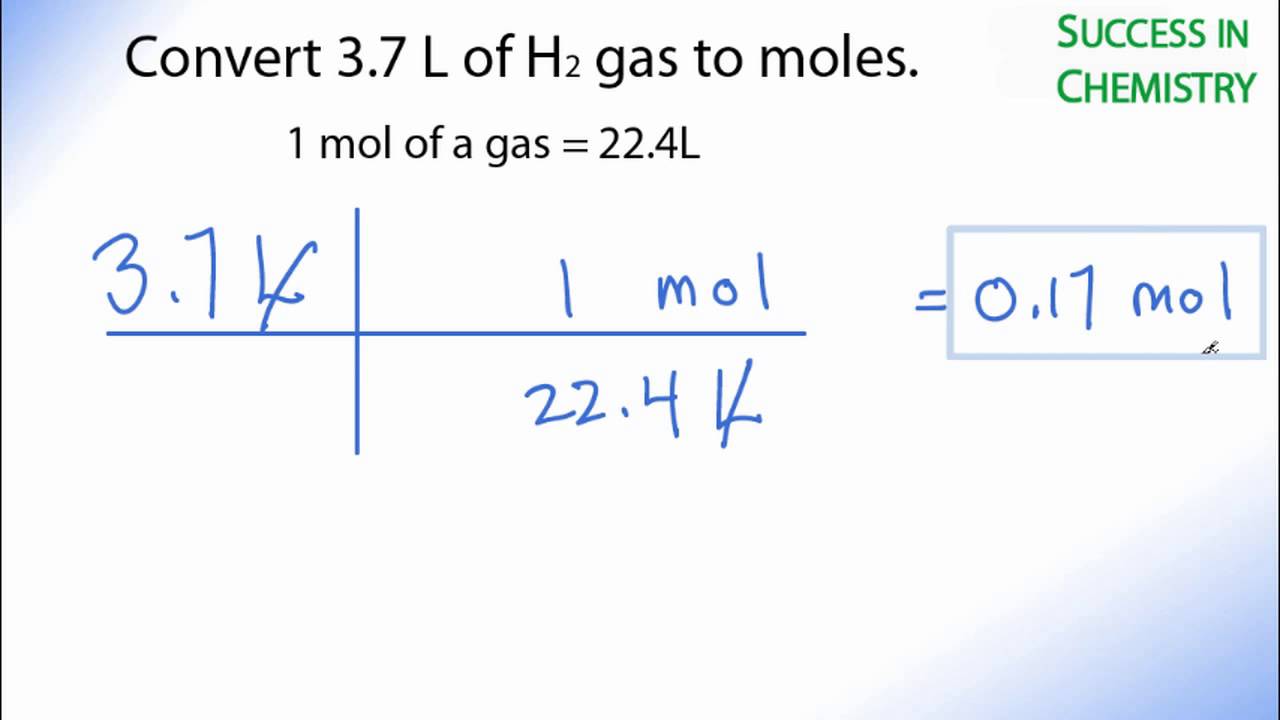
Convert 3.7 L of H2 Gas to Moles
Показать описание
In this video well learn to convert moles of H2 to liters. The technique used can be applied to any mole to liters conversion for ideal gases (like N2, O2...). For our practice problem we’ll convert 3.7 mole of H2 (Hydrogen gas) to liters. Using the same process in reverse we could also convert liters to moles.
For more help with moles to liters conversions and more:
Converting between moles and grams and liters is the cornerstone of being successful in stoichiometry, the study of chemical quantities. Take the time to learn mole conversions and you will find chemistry is much easier.
The use of conversion factors (also called factor-label method or dimensional analysis) is a more general technique for converting quantities. Once you understand how it works it can be applied to many different conversion (as long as you know the conversion factor).
For more help with moles to liters conversions and more:
Converting between moles and grams and liters is the cornerstone of being successful in stoichiometry, the study of chemical quantities. Take the time to learn mole conversions and you will find chemistry is much easier.
The use of conversion factors (also called factor-label method or dimensional analysis) is a more general technique for converting quantities. Once you understand how it works it can be applied to many different conversion (as long as you know the conversion factor).
Convert 3.7 L of H2 Gas to Moles
Convert Liters H2 Gas to Moles
How to Balance H2 + O2 = H2O
Replacing Lift Struts Quickly & Easily!
How to Balance Na + H2O = NaOH + H2 (Sodium plus Water)
Ultimate Duramax Conversion H2 Hummer Step 3 Johnny Magic 'Motorhead Messiah' Hline Conver...
How to Balance: N2 + H2 = NH3 (Synthesis of Ammonia)
Electrolysis: Producing hydrogen from water
IONIC EQUILIBRIUM in 1 Shot | Chemistry | 1st PUC
How to Predict Products of Chemical Reactions | How to Pass Chemistry
How to Balance H2 + Cl2 → HCl
What is the Chemical Name of Water?
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
How to Balance Fe + H2O = Fe3O4 + H2 (Iron + H2O in the form of steam)
Chemical reactions between metals and water
How to balance: Na + H2O = NaOH + H2
The Quencher H2.0 FlowState™ Tumbler
Water vs Battery || H2 and O2 out from water || Water Electrolysis Gas #science #experiment
How to Replace Brake Light Switch 2007-14 Chevy Silverado
How to program A Hummer H2 remote key fob 2003 - 2007
Why You Need To DO THIS TO YOUR CAR'S WEATHERSTRIPPING NOW!
5 Signs It's Time To Replace Your Spark Plugs
Hummer H2 2005 - Transmission Fluid Change #3
Problem 1 on Block Diagram Reduction
Комментарии
 0:01:37
0:01:37
 0:01:40
0:01:40
 0:00:59
0:00:59
 0:06:31
0:06:31
 0:02:02
0:02:02
 0:14:33
0:14:33
 0:01:00
0:01:00
 0:00:54
0:00:54
 4:52:15
4:52:15
 0:04:50
0:04:50
 0:01:22
0:01:22
 0:00:53
0:00:53
 0:25:16
0:25:16
 0:01:19
0:01:19
 0:01:01
0:01:01
 0:02:04
0:02:04
 0:00:31
0:00:31
 0:00:54
0:00:54
 0:02:00
0:02:00
 0:01:33
0:01:33
 0:03:53
0:03:53
 0:02:23
0:02:23
 0:11:29
0:11:29
 0:09:16
0:09:16