filmov
tv
Calculating Freezing Point of a Solution

Показать описание
Chapter 12 Slide 100 Example Calculation
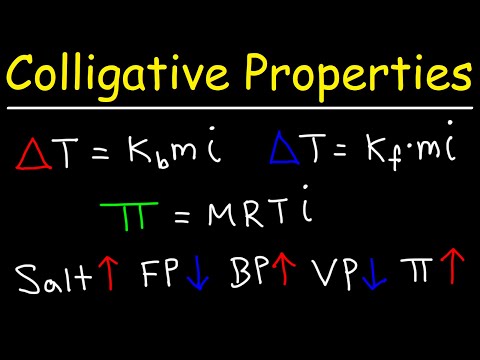
In the northeastern United States, during freezing weather CaCl2 is spread on icy highways to melt the ice. Calculate the freezing point lowering and freezing point of a solution containing 6.08 moles of CaCl2 in 0.500 kg of water.
In the northeastern United States, during freezing weather CaCl2 is spread on icy highways to melt the ice. Calculate the freezing point lowering and freezing point of a solution containing 6.08 moles of CaCl2 in 0.500 kg of water.
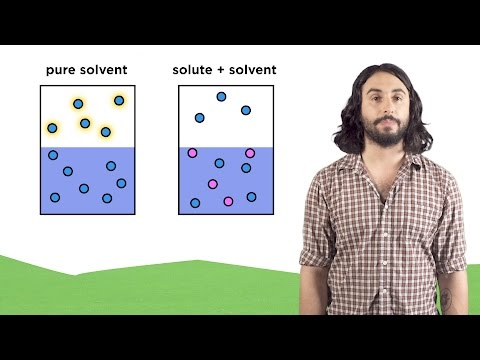
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
calculating freezing point of a solution
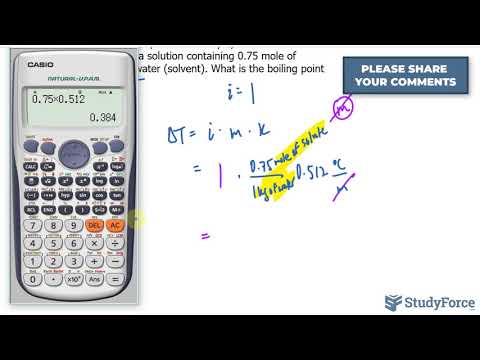
Calculate Freezing Point Depression
Freezing Point Depression Calculation
4 51 Boiling Point and Freezing Point (Calculations)
Calculating Freezing Point of a Solution
Calculating freezing point depression
Molality and Colligative Properties
HEAT | PART-2 | Class 9 Physics | AP TS State Board / CBSE | Manabadi Narayan Sir
Calculate the freezing point
ALEKS - Using a Solution Freezing Point to Calculate a Molar Mass
Freezing Point Depression Calculation
ALEKS: Using a solution freezing point to calculate molar mass
Calculating the Freezing Point of a Solution
Calculating Molar Mass from Freezing Point Depression
Calculate the Freezing Point Depression | Colligative Property #chemistry #science #shorts #short
Freezing Point Depression
solving for molar mass from freezing point depression
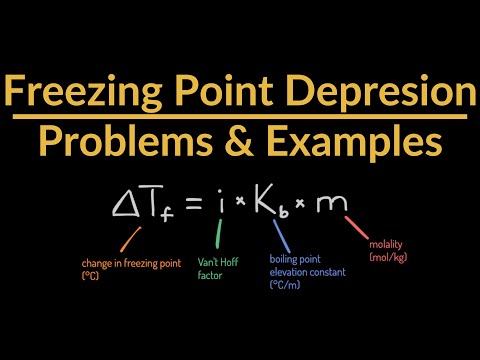
Freezing Point Depression Problems & Example (Colligative Property & Solving for New Freezin...
⚗️ Calculate the Freezing Point Depression
Depression in freezing point I Anti-freezing Properties #shorts #physics #chemistry #experiment
Determining Molar Mass of Unknown using Freezing Point Depression (Colligative Properties)
Calculating Freezing Point using Kf
Honors Chemistry Notes 11.6- Freezing Point / Boiling Point Calculations
Комментарии
 0:25:23
0:25:23
 0:03:39
0:03:39
 0:04:53
0:04:53
 0:01:22
0:01:22
 0:09:50
0:09:50
 0:04:25
0:04:25
 0:02:53
0:02:53
 0:05:10
0:05:10
 1:30:10
1:30:10
 0:01:57
0:01:57
 0:09:50
0:09:50
 0:03:29
0:03:29
 0:05:35
0:05:35
 0:06:10
0:06:10
 0:02:44
0:02:44
 0:01:00
0:01:00
 0:08:36
0:08:36
 0:03:37
0:03:37
 0:09:52
0:09:52
 0:02:37
0:02:37
 0:01:00
0:01:00
 0:04:41
0:04:41
 0:05:00
0:05:00
 0:11:30
0:11:30