filmov
tv
Electrolysis of Aqueous Solution | Redox Equilibrium

Показать описание
Electrolysis of Aqueous Solution
Form 5 Chemistry KSSM Chapter 1 Redox Equilibrium
Electrolysis is a process of decomposition of an electrolyte by an electric current.
An aqueous solution is produced when a solute is dissolved in water.
Form 5 Chemistry KSSM Chapter 1 Redox Equilibrium
Electrolysis is a process of decomposition of an electrolyte by an electric current.
An aqueous solution is produced when a solute is dissolved in water.
GCSE Chemistry - Electrolysis Part 3/3 - Aqueous Solutions
GCSE Chemistry Revision 'Electrolysis of Aqueous Solutions 1'
Electrolysis of Aqueous Solutions
R3.2.15 Electrolysis of aqueous solutions (HL)
GCSE Chemistry Revision 'Electrolysis of Aqueous Solutions 2'
Electrochemistry Gr 12 : Electrolysis of aqueous solutions
Electrolysis of an Aqueous Solution
R3.2.15 Predict and explain the products of electrolysis of aqueous solutions [HL IB Chemistry]
04.ELECTROCHEMI CLASS 12 CBSE CHSE IISER NISER NEET JEEMAIN OUAT ALLEN NEET CHEMISTRY MODULE SOLUTI
Electrolysis of Water - Electrochemistry
19.1 Electrolysis of aqueous solutions (HL)
Electrolysis of Salt Water - Aqueous Compounds | GCSE Chemistry
What Is Electrolysis | Reactions | Chemistry | FuseSchool
9 Electrolysis of Aqueous Solutions
Chemistry experiment 26 - Electrolysis of aqueous solutions
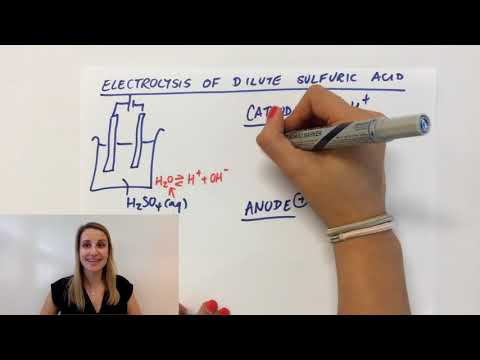
Explaining the electrolysis of dilute sulfuric acid H2SO4 (aq) GCSE Chemistry
Electrolysis of Aqueous Solutions GSCE Chemistry Science
Electrolysis of Aqueous Solution | Redox Equilibrium
GCSE - CHEMISTRY - ELECTRO CHEMISTRY - LESSON 3 - electrolysis of an aqueous solution
electrolysis of aqueous nacl using iron electrods #chemistry #ytshorts @crazyxyz
#electrolysis of aqueous solution of NaCl.. it's working..
Simple and easy science experiments.Electrolysis of water with NaCl.#shorts #diy #experiment #dwe .
GCSE Chemistry Electrolysis Quiz
How To Answer Any ELECTROLYSIS Question
Комментарии
 0:06:03
0:06:03
 0:04:42
0:04:42
 0:06:59
0:06:59
 0:03:47
0:03:47
 0:03:52
0:03:52
 0:10:10
0:10:10
 0:14:15
0:14:15
 0:05:37
0:05:37
 0:34:50
0:34:50
 0:13:12
0:13:12
 0:04:02
0:04:02
 0:03:14
0:03:14
 0:05:11
0:05:11
 0:15:11
0:15:11
 0:03:55
0:03:55
 0:03:01
0:03:01
 0:10:53
0:10:53
 0:07:06
0:07:06
 0:08:21
0:08:21
 0:00:27
0:00:27
 0:00:35
0:00:35
 0:00:15
0:00:15
 0:00:46
0:00:46
 0:08:47
0:08:47