filmov
tv
Saturated, Unsaturated and Super saturated solutions | ch#6 | 9th class chemistry

Показать описание
Saturated, Unsaturated and Super saturated solutions | ch#6 | 9th class chemistry
#9thclasschemistry
#Mpluschemistry
● 9th class chemistry lecture
● chapter number 6 (solution)
● punjab text book chemistry
● Matric Part-1 chemistry lectures
● Chemistry online lectures Chapter number 6 in 9th class chemistry punjab board.
● chemistry online lectures
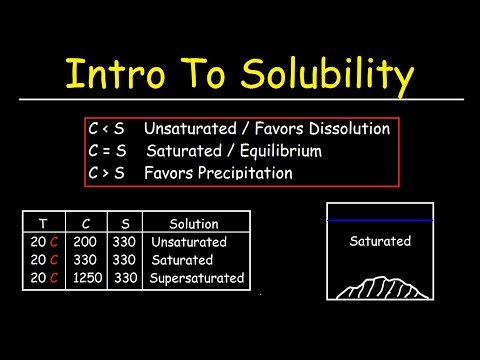
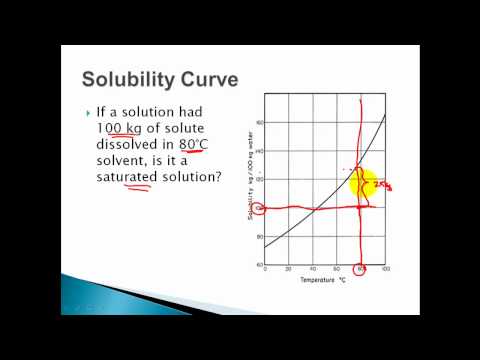
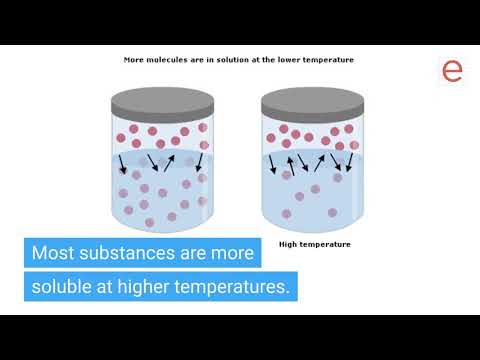
● Basic concepts of solutions SATURATED SOLUTION
A solution containing maximum amount of solute at a given temperature is called
saturated solution. On the particle level, a saturated solution is the one, in which
undissolved solute is in equilibrium with dissolved solute.
At this stage, dynamic equilibrium is established. Although dissolution and
crystallization continues at a given temperature, but the net amount of dissolved solute
remains constant.
6.2.1 Unsaturated Solution
A solution which contains lesser amount of solute than that which is required to
saturate it at a given temperature, is called unsaturated solution. Such solutions have
the capacity to dissolve more solute to become a saturated solution.
6.2.2 Supersaturated Solution
The solution that is more
concentrated than a saturated solution is known as supersaturated solution. Supersaturated solutions are not stable.
#9thclasschemistry
#Mpluschemistry
● 9th class chemistry lecture
● chapter number 6 (solution)
● punjab text book chemistry
● Matric Part-1 chemistry lectures
● Chemistry online lectures Chapter number 6 in 9th class chemistry punjab board.
● chemistry online lectures
● Basic concepts of solutions SATURATED SOLUTION
A solution containing maximum amount of solute at a given temperature is called
saturated solution. On the particle level, a saturated solution is the one, in which
undissolved solute is in equilibrium with dissolved solute.
At this stage, dynamic equilibrium is established. Although dissolution and
crystallization continues at a given temperature, but the net amount of dissolved solute
remains constant.
6.2.1 Unsaturated Solution
A solution which contains lesser amount of solute than that which is required to
saturate it at a given temperature, is called unsaturated solution. Such solutions have
the capacity to dissolve more solute to become a saturated solution.
6.2.2 Supersaturated Solution
The solution that is more
concentrated than a saturated solution is known as supersaturated solution. Supersaturated solutions are not stable.
Комментарии
 0:01:51
0:01:51
 0:15:17
0:15:17
 0:05:15
0:05:15
 0:08:17
0:08:17
 0:13:20
0:13:20
 0:01:27
0:01:27
 0:05:38
0:05:38
 0:04:38
0:04:38
 0:01:15
0:01:15
 0:05:24
0:05:24
 0:03:51
0:03:51
 0:00:16
0:00:16
 0:00:11
0:00:11
 0:17:32
0:17:32
 0:01:00
0:01:00
 0:02:16
0:02:16
 0:14:14
0:14:14
 0:01:59
0:01:59
 0:00:56
0:00:56
 0:14:05
0:14:05
 0:04:56
0:04:56
 0:01:48
0:01:48
 0:05:00
0:05:00
 0:10:30
0:10:30