filmov
tv
Carnot Cycle & Efficiency

Показать описание
Chapter: Carnot Cycle & Efficiency
Subject: Engineering Thermodynamics & Fluid Mechanics
Suitable for: 1st Year Engineering Students
Subject: Engineering Thermodynamics & Fluid Mechanics
Suitable for: 1st Year Engineering Students
Carnot Cycle & Heat Engines, Maximum Efficiency, & Energy Flow Diagrams Thermodynamics &am...
CARNOT CYCLE | Easy and Basic
Efficiency of Carnot Cycle
Carnot Cycle & Efficiency
Why We Can't Invent a Perfect Engine: Crash Course Engineering #10
The Carnot Cycle | Thermodynamics | (Solved Examples)
Carnot Cycle And Carnot Heat Engine - Efficiency of carnot cycle
Reversible Processes and CARNOT CYCLE in 12 Minutes!
Carnot Cycle - PV diagram, efficiency derivation and limitations [Hindi] #carnotcycle #carnot #cycle
CARNOT CYCLE: efficiency of carnot cycle
Carnot Cycle and Carnot Efficiency
Carnot Cycle Efficiency Derivation
Carnot Cycle - An Ideal Heat Engine
Carnot Cycle Efficiency and Net Power Output Calculations
Carnot cycle.Work . Efficiency. Isothermal and Adiabatic process. Adiabatic and Diathermal wall.
Thermodynamics Example 15b: Carnot Cycles
Why Carnot Cycle is Most Efficient? | YOLO JEE Advance Physics with Vikrant Kirar
Heat Engines, Thermal Efficiency, & Energy Flow Diagrams - Thermodynamics & Physics Problems
Carnot cycle and its efficiency...
Carnot heart engine class 11 | petrol engine class 11 | carnot cycle | Carnot theorem | carnot engin
Efficiency of the Carnot Cycle
Carnot cycle|Concept|How to derive efficiency of Carnot cycle|Animation|pv Ts diagram|GTU|Most Imp
Carnot cycle efficiency... bsc physics important topic.. hand written notes
Carnot Cycle and Efficiency
Комментарии
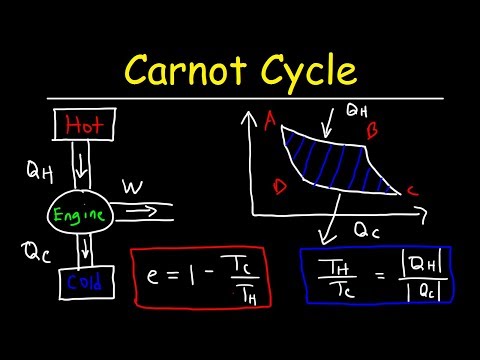 0:20:17
0:20:17
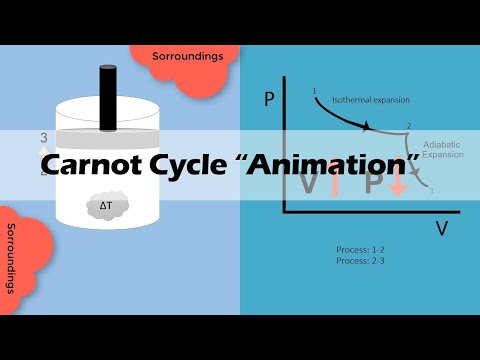 0:04:12
0:04:12
 0:03:45
0:03:45
 0:11:25
0:11:25
 0:12:55
0:12:55
 0:11:52
0:11:52
 0:24:56
0:24:56
 0:11:48
0:11:48
 0:08:27
0:08:27
 0:12:27
0:12:27
 0:23:04
0:23:04
 0:15:14
0:15:14
 0:04:40
0:04:40
 0:31:55
0:31:55
 0:07:34
0:07:34
 0:06:02
0:06:02
 0:10:03
0:10:03
 0:21:10
0:21:10
 0:16:25
0:16:25
 0:27:10
0:27:10
 0:38:30
0:38:30
 0:12:07
0:12:07
 0:00:14
0:00:14
 0:22:31
0:22:31