filmov
tv
Bohr's Model of Hydrogen Atom | Structure of Atom | Class 11th & 12th | Science

Показать описание
Welcome to this comprehensive video on Bohr's atomic model of hydrogen, a fundamental concept in chemistry that laid the foundation for our modern understanding of the atomic structure. As Class 11 students, understanding this concept is crucial for your academic success and will also give you a deeper appreciation for the fascinating world of atoms and molecules.
Follow me:
Instagram- @abhijeetchobey
Twitter- @abhijeetchobey
In this video, we'll delve into the life and work of Niels Bohr, a Danish physicist who revolutionized our understanding of the atomic structure. We'll explore how Bohr's model of the hydrogen atom challenged the existing Rutherford model and introduced the concept of energy levels, electron jumps, and wave-particle duality.
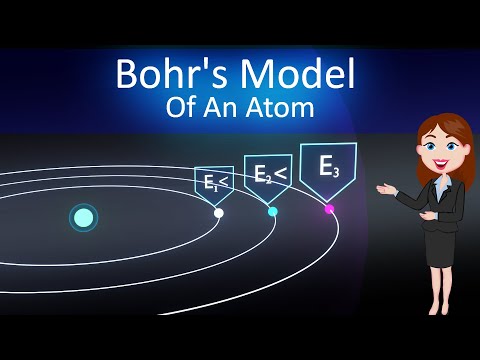
We'll start by discussing the limitations of the Rutherford model, which portrayed the atom as a miniature solar system with electrons orbiting the nucleus in fixed paths. Bohr, however, proposed that electrons occupy specific energy levels or shells around the nucleus, and that they jump from one energy level to another by emitting or absorbing energy in the form of photons.
We'll then dive deeper into the key features of Bohr's model, including:
- Energy levels: We'll explore how electrons occupy specific energy levels or shells, and how these energy levels are related to the electron's distance from the nucleus.
- Electron jumps: We'll discuss how electrons jump from one energy level to another, emitting or absorbing energy in the form of photons.
Throughout the video, we'll use engaging animations, diagrams, and examples to illustrate these complex concepts and make them easy to understand. We'll also provide plenty of examples and practice questions to help you reinforce your learning and feel confident in your understanding of Bohr's atomic model.
ALL PARTS OF THIS CHAPTER
Part 1 (Subatomic Particles)
Part 2 (Discovery of Electrons)
Part 3 (Charge to mass Ratio of Electron)
Part 4 (Discovery of Neutrons)
Part 5 (Discovery of Protons)
Part 6 (Thomson Model of Atom)
Part 7 (Rutherford's Model of Atom)
Part 8 (Rutherford's Model Pos. & Lim.)
Part 9 (Atomic Number & Mass Number)
Part 10 (Isobars & Isotopes)
Part 11 (Developments Leading to Bohr Model Atom)
Part 12 (Wave Nature of Electromagnetic Radiation)
Part 13 (Planck's Quantum Theory)
Part 14 (Photoelectric Effect Part-1)
Part 15 (Photoelectric Effect Part-2)
Part 16 (Dual Behaviour of E.M. Radiation)
Part 17 (Atomic Spectrum)
Part 18 (Emission & Absorption Spectrum)
Part 19 (Line Spectrum of Hydrogen Atom)
Part 20 (Bohr's Model of Hydrogen Atom)
#bohrsatomicmodel #nielsbohr
Follow me:
Instagram- @abhijeetchobey
Twitter- @abhijeetchobey
In this video, we'll delve into the life and work of Niels Bohr, a Danish physicist who revolutionized our understanding of the atomic structure. We'll explore how Bohr's model of the hydrogen atom challenged the existing Rutherford model and introduced the concept of energy levels, electron jumps, and wave-particle duality.
We'll start by discussing the limitations of the Rutherford model, which portrayed the atom as a miniature solar system with electrons orbiting the nucleus in fixed paths. Bohr, however, proposed that electrons occupy specific energy levels or shells around the nucleus, and that they jump from one energy level to another by emitting or absorbing energy in the form of photons.
We'll then dive deeper into the key features of Bohr's model, including:
- Energy levels: We'll explore how electrons occupy specific energy levels or shells, and how these energy levels are related to the electron's distance from the nucleus.
- Electron jumps: We'll discuss how electrons jump from one energy level to another, emitting or absorbing energy in the form of photons.
Throughout the video, we'll use engaging animations, diagrams, and examples to illustrate these complex concepts and make them easy to understand. We'll also provide plenty of examples and practice questions to help you reinforce your learning and feel confident in your understanding of Bohr's atomic model.
ALL PARTS OF THIS CHAPTER
Part 1 (Subatomic Particles)
Part 2 (Discovery of Electrons)
Part 3 (Charge to mass Ratio of Electron)
Part 4 (Discovery of Neutrons)
Part 5 (Discovery of Protons)
Part 6 (Thomson Model of Atom)
Part 7 (Rutherford's Model of Atom)
Part 8 (Rutherford's Model Pos. & Lim.)
Part 9 (Atomic Number & Mass Number)
Part 10 (Isobars & Isotopes)
Part 11 (Developments Leading to Bohr Model Atom)
Part 12 (Wave Nature of Electromagnetic Radiation)
Part 13 (Planck's Quantum Theory)
Part 14 (Photoelectric Effect Part-1)
Part 15 (Photoelectric Effect Part-2)
Part 16 (Dual Behaviour of E.M. Radiation)
Part 17 (Atomic Spectrum)
Part 18 (Emission & Absorption Spectrum)
Part 19 (Line Spectrum of Hydrogen Atom)
Part 20 (Bohr's Model of Hydrogen Atom)
#bohrsatomicmodel #nielsbohr
Комментарии
 0:04:50
0:04:50
 0:21:44
0:21:44
 0:05:05
0:05:05
 0:06:21
0:06:21
 0:08:24
0:08:24
 0:06:48
0:06:48
 0:03:37
0:03:37
 0:05:50
0:05:50
 2:35:35
2:35:35
 0:21:11
0:21:11
 0:03:47
0:03:47
 1:12:19
1:12:19
 0:02:10
0:02:10
 0:00:59
0:00:59
 0:11:52
0:11:52
 1:08:09
1:08:09
 0:04:38
0:04:38
 0:03:32
0:03:32
 0:37:02
0:37:02
 0:07:31
0:07:31
 0:00:20
0:00:20
 0:53:15
0:53:15
 1:00:02
1:00:02
 0:10:37
0:10:37