filmov
tv
The Ultraviolet Catastrophe: How does light really behave?

Показать описание
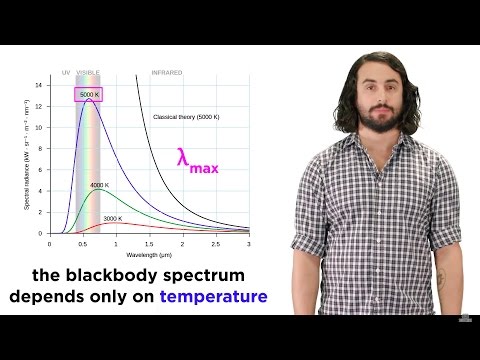
When objects get very hot, they emit lots of radiation but the classical theory predicted that hot objects would radiate infinite energy which they do not, therefore scientists had to revise physics.
First, Max Planck came along and proposed that if the radiated energy had been quantized (in energy chunks) as opposed to continuously distributed, then the theory would match the experiments. Planck proposed quantization but had no explanation for why this might be physically motivated.
This film was created by #WWU student Michael Dymek for the summer #SciComm course Phys/Comm 297 final project.
Students with little to no video editing experience or storytelling training turned into video producers in six weeks. We hope you enjoy this science story as much as we enjoyed making it. Spark Science host and instructor of this course, Dr. Barber DeGraaff, is very proud of each of them.
First, Max Planck came along and proposed that if the radiated energy had been quantized (in energy chunks) as opposed to continuously distributed, then the theory would match the experiments. Planck proposed quantization but had no explanation for why this might be physically motivated.
This film was created by #WWU student Michael Dymek for the summer #SciComm course Phys/Comm 297 final project.
Students with little to no video editing experience or storytelling training turned into video producers in six weeks. We hope you enjoy this science story as much as we enjoyed making it. Spark Science host and instructor of this course, Dr. Barber DeGraaff, is very proud of each of them.
The ULTRAVIOLET CATASTROPHE
What is the Ultraviolet Catastrophe?
Quantization of Energy Part 1: Blackbody Radiation and the Ultraviolet Catastrophe
The Ultraviolet Catastrophe Experiment
Max Planck Solves the Ultraviolet Catastrophe for Blackbody Radiation | Doc Physics
The Ultraviolet Catastrophe: How does light really behave?
Mystery of the Ultraviolet Catastrophe in Quantum Physics
The Ultraviolet Catastrophe (Part 1)
Quantum Mechanics | Blackbody Radiation Problems⚡| IIT JAM, CUET PG, JEST & TIFR 2026
I wish I was taught the birth of Quantum Mechanics this way!
The Ultraviolet Catastrophe
What is Ultraviolet Catastrophe - 3D Animation #physics #quantum
The Ultraviolet Catastrophe
Black Body 6 of 9; The Ultraviolet Catastrophe
COMPLETE - Blackbody, Ultraviolet Catastrophe & Planck Postulate | Birth of Quantum Mechanics
14.12 What was the Ultra violet catastrophe
No, Planck didn't solve the UV Catastrophe. Here is the real story!!
Neil deGrasse Tyson on the ultraviolet catastrophe and the birth of Quantum Mechanics #science
Ultraviolet Catastrophe of Blackbody Radiation
The Ultraviolet Catastrophe (Part 2)
Planck's Constant and The Origin of Quantum Mechanics | Space Time | PBS Digital Studios
Something Strange Happens When You Trust Quantum Mechanics
The Ultraviolet catastrophe - Preliminary Analysis
Atomic models and how quantum physics began: blackbody radiation and the ultraviolet catastrophe
Комментарии
 0:06:32
0:06:32
 0:40:29
0:40:29
 0:06:43
0:06:43
 0:08:02
0:08:02
 0:05:27
0:05:27
 0:04:22
0:04:22
 0:00:50
0:00:50
 0:11:14
0:11:14
 1:28:16
1:28:16
 0:21:48
0:21:48
 0:25:26
0:25:26
 0:00:33
0:00:33
 0:02:16
0:02:16
 0:09:33
0:09:33
 0:28:47
0:28:47
 0:04:13
0:04:13
 0:18:00
0:18:00
 0:00:53
0:00:53
 0:00:58
0:00:58
 0:18:32
0:18:32
 0:15:16
0:15:16
 0:33:01
0:33:01
 0:26:39
0:26:39
 0:19:44
0:19:44