filmov
tv
The Ultraviolet Catastrophe

Показать описание
the ultraviolet catastrophe.
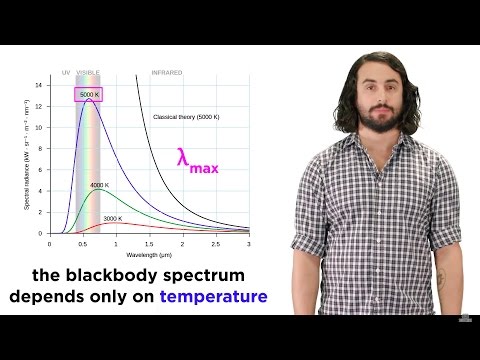
distribution of energy in black body radiation.
Imagine you have a hot object, like a piece of metal, and it's giving off light. Now, light is made up of tiny particles called photons. These photons have different amounts of energy depending on their color or frequency. Some photons have low energy, like red light, while others have higher energy, like blue or violet light.
Scientists in the past were trying to figure out how much energy this hot object would give off at different frequencies. They thought that as the frequency increased, the energy of the photons would also increase, but they ran into a problem.
According to their calculations using classical physics, the energy of the photons should keep increasing as the frequency gets higher and higher. This would mean that at really high frequencies, like in the ultraviolet part of the light spectrum, the energy would become infinitely large. That doesn't make sense because we can't have something with an infinite amount of energy.
Therefore, this problem where the energy kept getting bigger and bigger as the frequency increased was called the "ultraviolet catastrophe." It was a big puzzle for scientists because it didn't match what they observed in real life.
To solve this puzzle, a scientist named Max Planck came up with a new idea. He suggested that the energy of the photons is not continuous but comes in tiny packets called "quanta." It's like if you had a bowl of candies, and you could only take one candy at a time. You can't have half a candy or an infinite number of candies.
This idea of energy coming in small packets, or quanta, was the start of a new theory called quantum mechanics. It helped explain why the energy of the photons didn't keep increasing without limit. It also laid the foundation for the discoveries of other scientists, like Albert Einstein, who explained the behavior of light using the idea of photons.
The ultraviolet catastrophe was a problem that scientists faced when they tried to understand how much energy an object gives off as light at different frequencies. It led to the development of quantum mechanics, which is a really important part of physics that helps us understand how things work on a very tiny scale.
distribution of energy in black body radiation.
Imagine you have a hot object, like a piece of metal, and it's giving off light. Now, light is made up of tiny particles called photons. These photons have different amounts of energy depending on their color or frequency. Some photons have low energy, like red light, while others have higher energy, like blue or violet light.
Scientists in the past were trying to figure out how much energy this hot object would give off at different frequencies. They thought that as the frequency increased, the energy of the photons would also increase, but they ran into a problem.
According to their calculations using classical physics, the energy of the photons should keep increasing as the frequency gets higher and higher. This would mean that at really high frequencies, like in the ultraviolet part of the light spectrum, the energy would become infinitely large. That doesn't make sense because we can't have something with an infinite amount of energy.
Therefore, this problem where the energy kept getting bigger and bigger as the frequency increased was called the "ultraviolet catastrophe." It was a big puzzle for scientists because it didn't match what they observed in real life.
To solve this puzzle, a scientist named Max Planck came up with a new idea. He suggested that the energy of the photons is not continuous but comes in tiny packets called "quanta." It's like if you had a bowl of candies, and you could only take one candy at a time. You can't have half a candy or an infinite number of candies.
This idea of energy coming in small packets, or quanta, was the start of a new theory called quantum mechanics. It helped explain why the energy of the photons didn't keep increasing without limit. It also laid the foundation for the discoveries of other scientists, like Albert Einstein, who explained the behavior of light using the idea of photons.
The ultraviolet catastrophe was a problem that scientists faced when they tried to understand how much energy an object gives off as light at different frequencies. It led to the development of quantum mechanics, which is a really important part of physics that helps us understand how things work on a very tiny scale.
 0:06:32
0:06:32
 0:40:29
0:40:29
 0:06:43
0:06:43
 0:08:02
0:08:02
 0:21:48
0:21:48
 0:05:27
0:05:27
 0:28:47
0:28:47
 0:00:50
0:00:50
 0:01:17
0:01:17
 0:04:13
0:04:13
 0:18:00
0:18:00
 0:04:22
0:04:22
 0:34:08
0:34:08
 0:08:07
0:08:07
 0:25:41
0:25:41
 0:02:16
0:02:16
 0:08:45
0:08:45
 0:03:29
0:03:29
 0:02:57
0:02:57
 0:00:53
0:00:53
 0:09:33
0:09:33
 0:04:00
0:04:00
 0:00:57
0:00:57
 0:42:32
0:42:32