filmov
tv
Protein charge and isoelectric point (pI) - 'basic' vs 'acidic' etc.

Показать описание
Whether, in which direction, and to what extent, a protein is charged depends on “how it’s spelled” (what protein letters (amino acids) does it have in what order?) and what the pH is. pH is a measure of how many protons (H⁺) are in a solution - it’s an inverse log, so the more H⁺ (more acidic), the lower the pH. Some amino acids cab give protons (act as acids) or take protons, (act as bases) depending on the proton availability, & since those protons are positively-charged, give/take-ing them changes the protein’s charge. We can predict the overall charge of a protein using its isoelectric point (pI), which is the pH at which a protein is overall neutral. More “basic” proteins will have a higher pI and tend to be positively-charged and more “acidic” proteins have a lower pI and tend to be negatively charged.
⠀
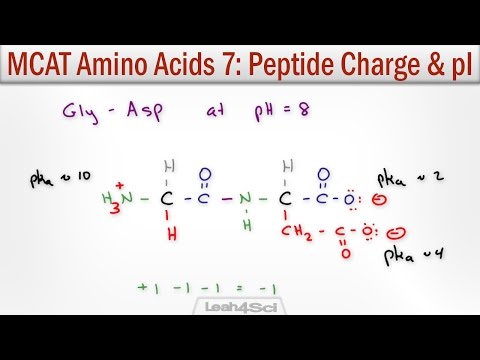
When you have free amino acids, they all have at least 2 groups that can give or take hydrogens. The amino end can exist as -NH₂ (neutral) or -NH₃⁺ (+ charged) & the carboxyl group can exist as -(C=O)-OH (neutral) or -(C=O)-O⁻ (- charged).
⠀
Physiological pH (the pH inside your body) is about 7.4, so most of the carboxyl groups are protonated & thus in the negatively-charged carboxylate form. But below 10, so most of the amino ends are in the +-charged NH₃⁺ form. So the charges cancel out – we call this a ZWITTERION⠀
⠀
When you link amino acids together, you’re only left with 1 amino end & one carboxyl end. So you only have to take those group’s protonation state into account once. But some of the side chains in the residues in between those ends can get protonated or deprotonated as well & when we talk about pKa’s for amino acids in the context of proteins or peptides, we usually are talking about the pKa of the side chain (if there is one). Sometimes this is referred to as the pKR or pK3.⠀
⠀
There are 2 side chains that are frequently deprotonated at physiological pH - we call these ACIDIC because they donate H⁺s & when they do they become negatively charged & now capable of accepting H⁺s (acting as a base) so we call them “conjugate bases.” The “acidic” refers to its NEUTRAL form being acidic.⠀
⠀
These are: Aspartic acid (Asp, D)(pKa ~3.65), which gives up a H⁺ to become aspartate & Glutamic acid (Glu, E)(pKa ~4.25) which gives up a H⁺ to become glutamate. Cysteine (Cys, C) & tyrosine (Tyr, Y) can can also deprotonate to give negatively charged chains, but do so much less readily - pKa of 8.4 for Cys & 10.5 for Tyr.⠀
⠀
There are 3 side chains that are frequently protonated at physiological pH - we call these “basic.” Lysine (Lys, K) (pKa ~ 10.28) & Arginine (Arg, R) (pKa ~13.2) are predominantly protonated at cellular pH, but Histidine (His, H) (5.97) is more “iffy” (remember pKa tells you when 1.2 the groups are deprotonated on average so you have a mix). Also, pKas are context-dependent so the pKa you get from a table is likely close to but not exactly the “real” pKa in the situation you’re looking at.⠀
⠀
At the protein level, we calculate the pI (isoelectric point), that pH at which the protein is neutral overall. It doesn’t mean that each individual amino acid is neutral, just that the non-neutral ones balance out.⠀
⠀
The higher the pI, the more H⁺-rich the protein is likely to be - you have to take it to a higher pH (more basic meaning less free H⁺) before it will shed H⁺s. We often refer to such proteins that are + charged at neutral pH as “basic” & they get that “basicity” because they have lots of basic residues (His, Lys, &/or Arg). They’re great for binding negatively-charged things like DNA or RNA.⠀
⠀
Proteins with pI’s below neutral are “acidic” and they usually have lots of Glu’s and Asp’s.⠀
in summary:- pH is a measure of free protons which are positively charged, but pH is on a negative log scale so LOWER pH means more positively-charged protons floating around
- some parts of proteins can grab onto them - the more there are (lower the pH) the more likely this is → increases charge (an make neutral side chains ➕ charged or ➖ charged side chains neutral)
- when pH is high, kiss those H⁺ goodbye! at high pH there aren’t many protons so this is less likely and some protein parts can actually donate them to the cause → decreases charge (➖ side chains can’t get neutralized (they stay ➖) And ➕ side chains have to “give up” their H⁺ (they get neutralized)
- proteins have different combinations of parts that give & parts that take
- as a result, a protein has an overall charge that depends on the pH⠀
Each protein has a specific point at which it at which the protein OVERALL is NEUTRAL - there can be ➕ & ➖ charged chains, but they perfectly cancel each other out & we call this charged but neutral condition ZWITTERIONIC. The pH at which it occurs the pI or ISOELECTRIC POINT
- Above the pI, the protein is ➖
- Below the pI, the protein is ➕
note: that structure is 4f3t, Elkayam et al.
⠀
When you have free amino acids, they all have at least 2 groups that can give or take hydrogens. The amino end can exist as -NH₂ (neutral) or -NH₃⁺ (+ charged) & the carboxyl group can exist as -(C=O)-OH (neutral) or -(C=O)-O⁻ (- charged).
⠀
Physiological pH (the pH inside your body) is about 7.4, so most of the carboxyl groups are protonated & thus in the negatively-charged carboxylate form. But below 10, so most of the amino ends are in the +-charged NH₃⁺ form. So the charges cancel out – we call this a ZWITTERION⠀
⠀
When you link amino acids together, you’re only left with 1 amino end & one carboxyl end. So you only have to take those group’s protonation state into account once. But some of the side chains in the residues in between those ends can get protonated or deprotonated as well & when we talk about pKa’s for amino acids in the context of proteins or peptides, we usually are talking about the pKa of the side chain (if there is one). Sometimes this is referred to as the pKR or pK3.⠀
⠀
There are 2 side chains that are frequently deprotonated at physiological pH - we call these ACIDIC because they donate H⁺s & when they do they become negatively charged & now capable of accepting H⁺s (acting as a base) so we call them “conjugate bases.” The “acidic” refers to its NEUTRAL form being acidic.⠀
⠀
These are: Aspartic acid (Asp, D)(pKa ~3.65), which gives up a H⁺ to become aspartate & Glutamic acid (Glu, E)(pKa ~4.25) which gives up a H⁺ to become glutamate. Cysteine (Cys, C) & tyrosine (Tyr, Y) can can also deprotonate to give negatively charged chains, but do so much less readily - pKa of 8.4 for Cys & 10.5 for Tyr.⠀
⠀
There are 3 side chains that are frequently protonated at physiological pH - we call these “basic.” Lysine (Lys, K) (pKa ~ 10.28) & Arginine (Arg, R) (pKa ~13.2) are predominantly protonated at cellular pH, but Histidine (His, H) (5.97) is more “iffy” (remember pKa tells you when 1.2 the groups are deprotonated on average so you have a mix). Also, pKas are context-dependent so the pKa you get from a table is likely close to but not exactly the “real” pKa in the situation you’re looking at.⠀
⠀
At the protein level, we calculate the pI (isoelectric point), that pH at which the protein is neutral overall. It doesn’t mean that each individual amino acid is neutral, just that the non-neutral ones balance out.⠀
⠀
The higher the pI, the more H⁺-rich the protein is likely to be - you have to take it to a higher pH (more basic meaning less free H⁺) before it will shed H⁺s. We often refer to such proteins that are + charged at neutral pH as “basic” & they get that “basicity” because they have lots of basic residues (His, Lys, &/or Arg). They’re great for binding negatively-charged things like DNA or RNA.⠀
⠀
Proteins with pI’s below neutral are “acidic” and they usually have lots of Glu’s and Asp’s.⠀
in summary:- pH is a measure of free protons which are positively charged, but pH is on a negative log scale so LOWER pH means more positively-charged protons floating around
- some parts of proteins can grab onto them - the more there are (lower the pH) the more likely this is → increases charge (an make neutral side chains ➕ charged or ➖ charged side chains neutral)
- when pH is high, kiss those H⁺ goodbye! at high pH there aren’t many protons so this is less likely and some protein parts can actually donate them to the cause → decreases charge (➖ side chains can’t get neutralized (they stay ➖) And ➕ side chains have to “give up” their H⁺ (they get neutralized)
- proteins have different combinations of parts that give & parts that take
- as a result, a protein has an overall charge that depends on the pH⠀
Each protein has a specific point at which it at which the protein OVERALL is NEUTRAL - there can be ➕ & ➖ charged chains, but they perfectly cancel each other out & we call this charged but neutral condition ZWITTERIONIC. The pH at which it occurs the pI or ISOELECTRIC POINT
- Above the pI, the protein is ➖
- Below the pI, the protein is ➕
note: that structure is 4f3t, Elkayam et al.
Комментарии
 0:12:45
0:12:45
 0:22:54
0:22:54
 0:14:26
0:14:26
 0:09:01
0:09:01
 0:29:43
0:29:43
 0:03:15
0:03:15
 0:02:57
0:02:57
 0:03:54
0:03:54
 0:09:04
0:09:04
 0:19:11
0:19:11
 0:18:16
0:18:16
 0:03:37
0:03:37
 0:04:01
0:04:01
 0:03:46
0:03:46
 0:03:02
0:03:02
 0:02:17
0:02:17
 0:03:10
0:03:10
 0:00:26
0:00:26
 0:21:39
0:21:39
 0:19:58
0:19:58
 0:24:25
0:24:25
 0:14:50
0:14:50
 0:04:58
0:04:58
 0:04:56
0:04:56