filmov
tv
Basics of Chemistry: Units of measurement in chemistry
Показать описание
Basics of Chemistry: Units of measurement in chemistry
In this video, we are going to look at units of measurement in chemistry. More specifically, you are going to learn about SI Units, derived units, and metric prefixes.
It important to know that all measurements will have a number in a unit. You would never say I have 3. 3 what? This always need to be clarified. For example, if you had 70 kilograms of something, you would need to say I have 70 kg, not I have 70.
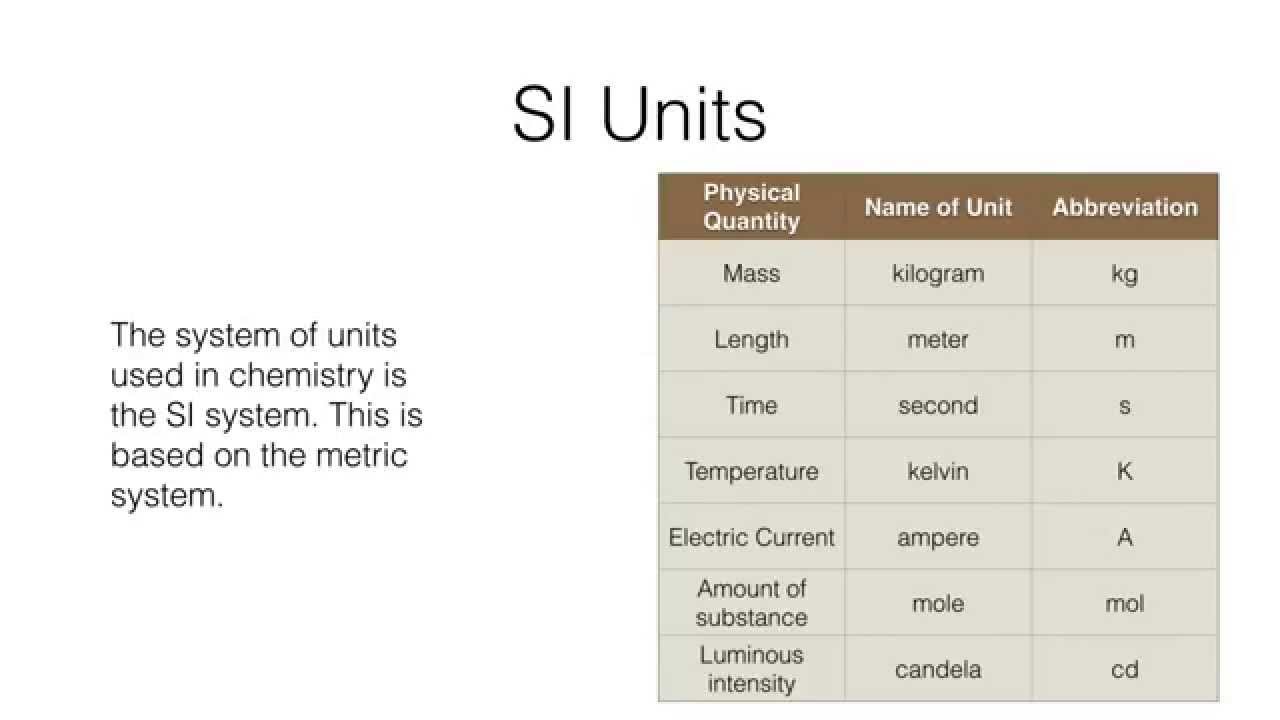
The SI system, from the international system of units, used base units as the basis for all units. These units are listed in the table and include kilogram for mass, meter’s for length, seconds for time, kelvin for temperature, ampere for electrical current, mole for the amount of something, can candela for luminous intensity. You might notice that the base unit for mass is the only one with a metric prefix.
When you combine one or more base units, you get derived units, or units that are made of more than one base unit. You may be familiar with many of these, such as density, which is mass divided by volume, or velocity, which is length, divided by time. Volume is also a derived unit, which comes from length cubed. 1cm cubed is equivalent to 1 mL. The joule, which is unit of energy, is equivalent to kilograms times meters squared over seconds squared.
There are quite a wide variety of metric prefixes that can be added in front of either the base units or derived units as you can see from the table here. Do you really have to know all of these? Most likely no. In fact, if you are just beginning your chemistry journey, I recommend learning only the following. Kilo, centi, milli, micro, and nano.
By placing the prefix in front of a base unit, that means you have a certain number of base units. For example, kilo means 1000, or 10 to the third power. Therefore a kilo unit, such as a kilogram means you have 1000 grams. Instead of writing out kilogram or millisecond, or nanogram, or any other number of possible combinations, these can be abbreviated by using the symbols for the prefix and base unit. A few other examples are seen here. 1 km is equal to 1000m, 10 cs = 0.01 s, and 345 micrograms = 0.000345 g.
In this lesion, you learned about si units, derived units, and metric prefixes. If you have any questions, leave a comment below or feel free to message me. Thanks for watching.
In this video, we are going to look at units of measurement in chemistry. More specifically, you are going to learn about SI Units, derived units, and metric prefixes.
It important to know that all measurements will have a number in a unit. You would never say I have 3. 3 what? This always need to be clarified. For example, if you had 70 kilograms of something, you would need to say I have 70 kg, not I have 70.
The SI system, from the international system of units, used base units as the basis for all units. These units are listed in the table and include kilogram for mass, meter’s for length, seconds for time, kelvin for temperature, ampere for electrical current, mole for the amount of something, can candela for luminous intensity. You might notice that the base unit for mass is the only one with a metric prefix.
When you combine one or more base units, you get derived units, or units that are made of more than one base unit. You may be familiar with many of these, such as density, which is mass divided by volume, or velocity, which is length, divided by time. Volume is also a derived unit, which comes from length cubed. 1cm cubed is equivalent to 1 mL. The joule, which is unit of energy, is equivalent to kilograms times meters squared over seconds squared.
There are quite a wide variety of metric prefixes that can be added in front of either the base units or derived units as you can see from the table here. Do you really have to know all of these? Most likely no. In fact, if you are just beginning your chemistry journey, I recommend learning only the following. Kilo, centi, milli, micro, and nano.
By placing the prefix in front of a base unit, that means you have a certain number of base units. For example, kilo means 1000, or 10 to the third power. Therefore a kilo unit, such as a kilogram means you have 1000 grams. Instead of writing out kilogram or millisecond, or nanogram, or any other number of possible combinations, these can be abbreviated by using the symbols for the prefix and base unit. A few other examples are seen here. 1 km is equal to 1000m, 10 cs = 0.01 s, and 345 micrograms = 0.000345 g.
In this lesion, you learned about si units, derived units, and metric prefixes. If you have any questions, leave a comment below or feel free to message me. Thanks for watching.
Комментарии
 0:18:49
0:18:49
 0:11:24
0:11:24
 0:02:49
0:02:49
 0:07:45
0:07:45
 0:18:14
0:18:14
 0:06:21
0:06:21
 0:08:54
0:08:54
 0:16:29
0:16:29
 0:00:22
0:00:22
 0:25:47
0:25:47
 0:24:18
0:24:18
 0:47:05
0:47:05
 0:39:45
0:39:45
 0:52:09
0:52:09
 1:04:57
1:04:57
 0:11:46
0:11:46
 1:08:40
1:08:40
 0:30:31
0:30:31
 0:23:08
0:23:08
 0:37:19
0:37:19
 0:11:53
0:11:53
 1:04:45
1:04:45
 0:09:06
0:09:06
 0:12:59
0:12:59