filmov
tv
How to Find the Empirical Formula of C6H12O6

Показать описание
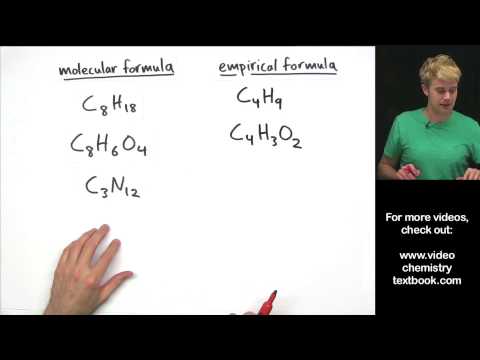
When given a molecular formula, look at the subscripts on all the different elements.
(If an element does not have a subscript, it is 1. For example, H2O has two hydrogens and one oxygen).
If you can divide ALL the subscripts by one number and still be left with all whole numbers, do that division. You are finding the greatest common factor (GCF).
The empirical formula is the formula with your subscripts as reduced as possible.
Note: There is not necessarily a real molecule containing the number of atoms shown in the empirical formula. It only shows the ratios of the atoms relative to each other.
(If an element does not have a subscript, it is 1. For example, H2O has two hydrogens and one oxygen).
If you can divide ALL the subscripts by one number and still be left with all whole numbers, do that division. You are finding the greatest common factor (GCF).
The empirical formula is the formula with your subscripts as reduced as possible.
Note: There is not necessarily a real molecule containing the number of atoms shown in the empirical formula. It only shows the ratios of the atoms relative to each other.
How to calculate Empirical Formula? 3 Easy Steps
Empirical Formula & Molecular Formula Determination From Percent Composition
Finding and Calculating an Empirical Formula of a Compound | How to Pass Chemistry
Writing Empirical Formula Practice Problems
How to Find the Empirical Formula of C6H12O6
Empirical Formula and Molecular Formula Introduction
How To Find The Empirical Formula of a Hydrated Ionic Compound
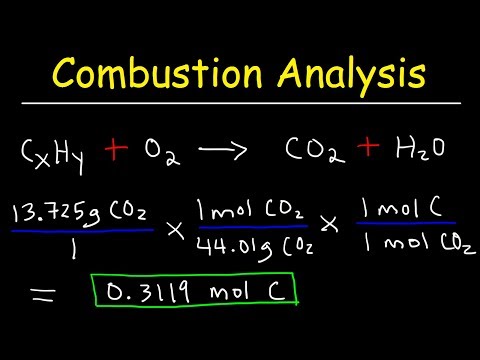
CHEMISTRY 101: Finding Empirical Formula Using Combustion Analysis for a Compound with C, H, O
Large-scale dynamics of equity markets of variable size: an empirical analysis
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems
trick to calculate Empirical formula✍️ | chemistry class 9th 10th 11th #empiricalformula #shorts
How to Find the Empirical Formula and molecular formula from percentage | Easy steps
Grade 11 Chemistry Tutorial - How to Find the Empirical Formula
Empirical Formula Grade 11 Exam
Calculating Molecular Formula from Empirical Formula
How to Find Empirical Formula - Empirical Intro - Five Steps
Worked example: Determining an empirical formula from percent composition data | Khan Academy
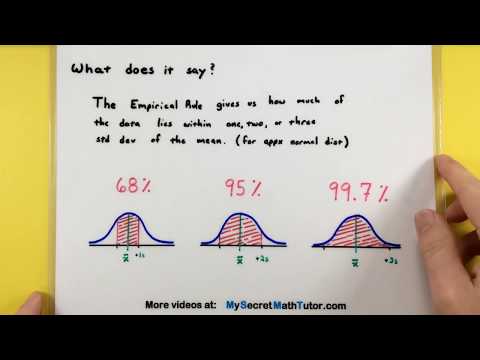
Statistics - How to use the Empirical Rule
S3E3 - How to Find Empirical Formula and Molecular Formula
Calculating the Empirical Formula From Unit Cell
Determine the empirical formula of a compound that contains 40.0% C, 6.7% H, and 53.3% O by mass.
Empirical Formulae From Percentage Composition | Chemical Calculations | Chemistry | FuseSchool
Empirical Formula: How to calculate | Stoichiometry | Chemistry
Empirical Formula and Molecular Formula | Basic Concept | Numerical Problems
Комментарии
 0:05:01
0:05:01
 0:11:00
0:11:00
 0:02:52
0:02:52
 0:06:09
0:06:09
 0:00:52
0:00:52
 0:08:31
0:08:31
 0:05:33
0:05:33
 0:04:12
0:04:12
 0:53:05
0:53:05
 0:16:49
0:16:49
 0:01:00
0:01:00
 0:06:58
0:06:58
 0:00:59
0:00:59
 0:05:08
0:05:08
 0:09:09
0:09:09
 0:09:07
0:09:07
 0:04:35
0:04:35
 0:05:37
0:05:37
 0:11:44
0:11:44
 0:04:14
0:04:14
 0:04:08
0:04:08
 0:04:33
0:04:33
 0:08:50
0:08:50
 0:13:30
0:13:30