filmov
tv
Determine the empirical formula of a compound that contains 40.0% C, 6.7% H, and 53.3% O by mass.

Показать описание
Determine the empirical formula of a compound that contains 40.0% C, 6.7% H, and 53.3% O by mass.
Empirical Formula & Molecular Formula Determination From Percent Composition
How to calculate Empirical Formula? 3 Easy Steps
Finding and Calculating an Empirical Formula of a Compound | How to Pass Chemistry
How to Find the Empirical Formula of C6H12O6
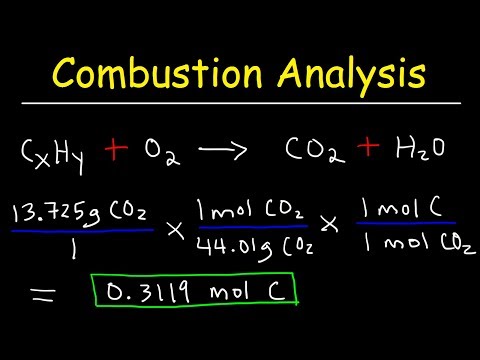
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems
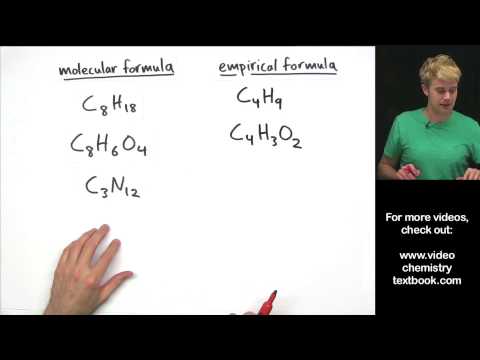
Empirical Formula and Molecular Formula Introduction
Writing Empirical Formula Practice Problems
Determine empirical formula given percentage components by mass
LV 20220716 Friction and Terminal Speed | Physics Aptitude Test (PAT) of Oxford University
CHEMISTRY 101: Finding Empirical Formula Using Combustion Analysis for a Compound with C, H, O
Empirical Formula: How to calculate | Stoichiometry | Chemistry
trick to calculate Empirical formula✍️ | chemistry class 9th 10th 11th #empiricalformula #shorts
Empirical Formula Grade 11 Exam
How To Calculate Empirical Formula|Super Trick|#shorts
Empirical Formula | Worked Example
Calculating Molecular Formula from Empirical Formula
Empirical and molecular formula grade 11
Grade 11 Chemistry Tutorial - How to Find the Empirical Formula
Empirical Formula - How to Determine | Positive Chemistry
Empirical Formula and Molecular Formula | Basic Concept | Numerical Problems
Determine the Empirical Formula
Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.
Calculating the Empirical Formula From Unit Cell
How To Find The Empirical Formula of a Hydrated Ionic Compound
Комментарии
 0:11:00
0:11:00
 0:05:01
0:05:01
 0:02:52
0:02:52
 0:00:52
0:00:52
 0:16:49
0:16:49
 0:08:31
0:08:31
 0:06:09
0:06:09
 0:01:00
0:01:00
 1:01:29
1:01:29
 0:04:12
0:04:12
 0:08:50
0:08:50
 0:01:00
0:01:00
 0:05:08
0:05:08
 0:00:17
0:00:17
 0:00:33
0:00:33
 0:09:09
0:09:09
 0:13:01
0:13:01
 0:00:59
0:00:59
 0:04:08
0:04:08
 0:13:30
0:13:30
 0:01:00
0:01:00
 0:06:55
0:06:55
 0:04:14
0:04:14
 0:05:33
0:05:33