filmov
tv
Part-1. Electronic configuration | ch#5 | 11th class Chemistry

Показать описание
Electronic configuration
Hunds rule for Electronic configuration
Pauli exclusion principle of Electronic configuration
Aufbau principle of Electronic configuration
#11thclasschemistry
#mjdchemistry
#Electronicconfiguration
Aufbau Principle
The electrons should be illed in energy
subshells in order of increasing energy values.
The electrons are irst placed in Is, 2s, 2p and
soon.
Pauli’s Exclusion Principle
This principle can be stated as follows:
It is impossible for two electrons residing
in the same orbital of a poly-electron atom to
have the same values of four quantum num bers, or Two electrons in the same orbital
should have opposite spins (↓↑ ).
Hund’s Rules
If, degenerate orbitals are available and more than one electrons are to be placed in them,
they should be placed in separate orbitals with the same spin rather than putting them in the same
orbital with opposite spins
Hunds rule for Electronic configuration
Pauli exclusion principle of Electronic configuration
Aufbau principle of Electronic configuration
#11thclasschemistry
#mjdchemistry
#Electronicconfiguration
Aufbau Principle
The electrons should be illed in energy
subshells in order of increasing energy values.
The electrons are irst placed in Is, 2s, 2p and
soon.
Pauli’s Exclusion Principle
This principle can be stated as follows:
It is impossible for two electrons residing
in the same orbital of a poly-electron atom to
have the same values of four quantum num bers, or Two electrons in the same orbital
should have opposite spins (↓↑ ).
Hund’s Rules
If, degenerate orbitals are available and more than one electrons are to be placed in them,
they should be placed in separate orbitals with the same spin rather than putting them in the same
orbital with opposite spins
Part-1. Electronic configuration | ch#5 | 11th class Chemistry
Electron Configuration - Basic introduction
CH 5 CHEMISTRY ELECTRON CONFIGURATIONS
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Part-2. Electronic configuration | ch#5 | 11th class Chemistry
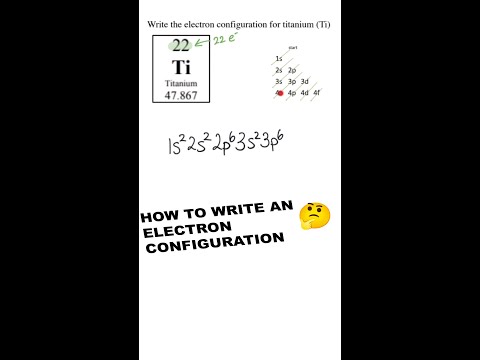
How to Write an Electron Configuration #chemistry #shorts #science #education #homework
Electron Configuration Diagrams | Properties of Matter | Chemistry | FuseSchool
DK 014 - Chap 5 - Electronic Configuration
GOC | Day 3 | Complete Isomerism | One Shot in English | JEE Main & Advanced
Electronic configuration electronics configuration of elements #short tricks
Tricks to learn Electronic Configuration #chemistry #class12 #easyway #subscribers #youtuber #viral
Electronic Configuration Best Trick 🥰🥰
What is Electronic Configuration? Electronic Configuration Of First Eighteen Elements ..Class 9th
A satisfying chemical reaction
How To do Electronic Configuration || Atomic Structure 08 || Electronic Configuration ||spdf
Sodium metal, soft, reactive, and squishy
Alakh Pandey Sir wife #shorts #alakhpandey #physicswallah
11th Class Chemistry, ch 5 - Electronic Distribution - FSc Chemistry Book 1
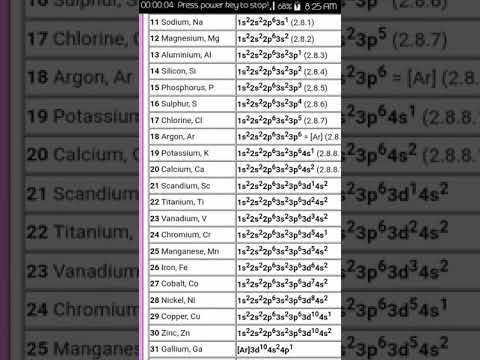
DK014 Chapter 5 How to write Electronic Configuration using spdf notation
Matric part 1 Chemistry, Electronic Configuration - Ch 2 Structure of Atoms - 9th Class
SUNIL SIR'S EASY WAY TO LEARN ATOMIC NUMBER#PHYSICSWALLAH
Why do we blink? #shorts
Best Teacher of Science Proved 🔥||Prashant kirad||NextToppers|#emotions #cbse #science #class10 #god...
PERIODIC TABLE BHUL GAYE।।SO SAD 😭😭😭। IAS INTERVIEW। IAS LOVER।
Комментарии
 0:24:08
0:24:08
 0:10:19
0:10:19
 0:14:44
0:14:44
 0:08:42
0:08:42
 0:21:50
0:21:50
 0:01:00
0:01:00
 0:04:59
0:04:59
 0:07:35
0:07:35
 4:04:22
4:04:22
 0:00:50
0:00:50
 0:00:29
0:00:29
 0:00:12
0:00:12
 0:00:16
0:00:16
 0:00:19
0:00:19
 0:13:36
0:13:36
 0:00:50
0:00:50
 0:00:16
0:00:16
 0:10:19
0:10:19
 0:06:03
0:06:03
 0:27:37
0:27:37
 0:00:16
0:00:16
 0:00:21
0:00:21
 0:00:23
0:00:23
 0:00:23
0:00:23