filmov
tv
Using Model-Based Meta-Analysis to Improve Decision-Making in Drug Development

Показать описание
Making the right choices in drug development often means the difference between getting a new medication to patients and it ending up in the scrap heap of failed programs. There is a surfeit of publicly available information on approved drugs as well as those currently in development. How can sponsors turn clinical trial data into understanding that helps chart the course for investigational drugs?
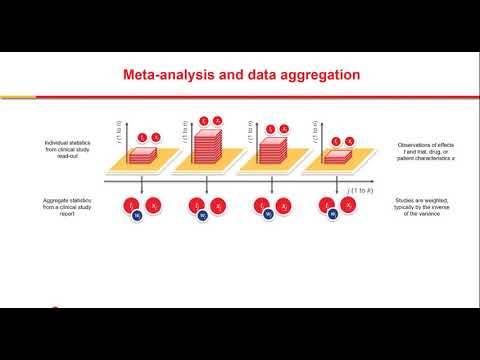
Model-based meta-analysis (MBMA) is an emerging methodology that quantifies clinical trial efficacy, tolerability, and safety information to enable strategic drug development decisions. The strategy involves a systematic search and tabulation of summary results from public sources which may be combined with proprietary clinical trial data. These data are then analyzed using nonlinear regression models which characterize the impacts of drug class, drug, dose, and time on the response(s) of interest.
In this webinar, Dr. Leon Bax presented several case studies that illustrate how MBMA was used to help sponsors:
- Position a drug within the competitive landscape
- Optimize clinical trial design
- Inform portfolio and marketing decisions to ensure commercial success
Model-based meta-analysis (MBMA) is an emerging methodology that quantifies clinical trial efficacy, tolerability, and safety information to enable strategic drug development decisions. The strategy involves a systematic search and tabulation of summary results from public sources which may be combined with proprietary clinical trial data. These data are then analyzed using nonlinear regression models which characterize the impacts of drug class, drug, dose, and time on the response(s) of interest.
In this webinar, Dr. Leon Bax presented several case studies that illustrate how MBMA was used to help sponsors:
- Position a drug within the competitive landscape
- Optimize clinical trial design
- Inform portfolio and marketing decisions to ensure commercial success
 0:45:33
0:45:33
 1:00:21
1:00:21
 0:33:21
0:33:21
 0:58:04
0:58:04
 0:22:41
0:22:41
 0:57:46
0:57:46
 0:43:51
0:43:51
 0:00:48
0:00:48
 1:03:33
1:03:33
 0:10:27
0:10:27
 0:08:15
0:08:15
 0:06:00
0:06:00
 0:03:49
0:03:49
 0:01:47
0:01:47
 0:15:44
0:15:44
 0:32:35
0:32:35
 0:09:43
0:09:43
 0:01:37
0:01:37
 0:34:50
0:34:50
 1:19:07
1:19:07
 0:03:43
0:03:43
 0:09:16
0:09:16
 0:14:18
0:14:18
 0:18:24
0:18:24