filmov
tv
Model-based meta-analysis for comparative efficacy and safety of therapeutics for COVID-19

Показать описание
Certara is launching the COVID-19 Clinical Outcomes Database, funded by the COVID-19 Therapeutics Accelerator. The Database includes observational studies and clinical trials with more than 10 patients that evaluate specific treatment options to improve disease outcome. It captures all patient characteristics, safety and efficacy outcomes, biomarkers and viral load data. The database also includes studies that evaluate patient characteristics stratified by disease outcome such as death, or progression to severe or critical disease and studies that report the time course of viral load, vital signs, and biomarkers.
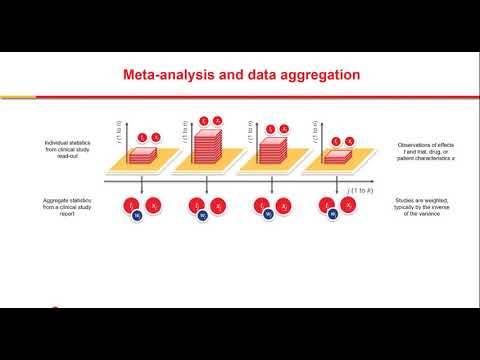
The Database can be applied to quantify comparative efficacy and safety of potential treatment options for COVID-19 and quickly interpret new reported outcomes in light of existing evidence. We can also mine the data to understand risk factors for disease severity, death, and duration of hospitalization. With model-based meta-analysis (MBMA), we can adjust for patient population differences that could impact the absolute or relative effect of treatments and provide a better estimate of the comparative treatment effect. Furthermore, MBMA can be used to quantify the longitudinal relationships between viral load, vital signs, biomarkers, and outcomes.
MBMA is particularly valuable for COVID-19, where we can bridge across many studies, thereby enabling comparison of treatments that were never tested together in the same clinical trial.
In this webinar, we discussed:
How to view and analyze the COVID-19 Clinical Outcomes Database using the CODEx web-based graphical interface
How MBMA can elucidate understanding of comparative safety and efficacy of COVID-19 therapeutics
Early insights from meta-analyses of COVID-19 clinical studies to date
The Database can be applied to quantify comparative efficacy and safety of potential treatment options for COVID-19 and quickly interpret new reported outcomes in light of existing evidence. We can also mine the data to understand risk factors for disease severity, death, and duration of hospitalization. With model-based meta-analysis (MBMA), we can adjust for patient population differences that could impact the absolute or relative effect of treatments and provide a better estimate of the comparative treatment effect. Furthermore, MBMA can be used to quantify the longitudinal relationships between viral load, vital signs, biomarkers, and outcomes.
MBMA is particularly valuable for COVID-19, where we can bridge across many studies, thereby enabling comparison of treatments that were never tested together in the same clinical trial.
In this webinar, we discussed:
How to view and analyze the COVID-19 Clinical Outcomes Database using the CODEx web-based graphical interface
How MBMA can elucidate understanding of comparative safety and efficacy of COVID-19 therapeutics
Early insights from meta-analyses of COVID-19 clinical studies to date
 0:45:33
0:45:33
 0:22:41
0:22:41
 0:33:21
0:33:21
 1:00:21
1:00:21
 0:58:04
0:58:04
 0:04:24
0:04:24
 0:01:59
0:01:59
 1:03:33
1:03:33
 0:42:36
0:42:36
 0:02:37
0:02:37
 0:10:03
0:10:03
 0:08:47
0:08:47
 0:03:29
0:03:29
 0:02:33
0:02:33
 1:02:35
1:02:35
 0:00:32
0:00:32
 0:07:18
0:07:18
 0:14:08
0:14:08
 0:58:42
0:58:42
 0:21:36
0:21:36
 1:00:39
1:00:39
 0:34:50
0:34:50
 0:01:42
0:01:42