filmov
tv
Hypertonic | Isotonic | Hypotonic Solution | Simple Explanation of Hypertonic Through Animation

Показать описание
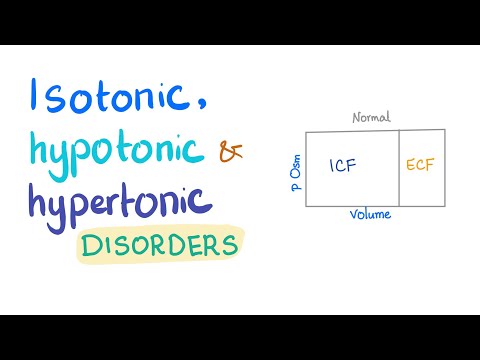
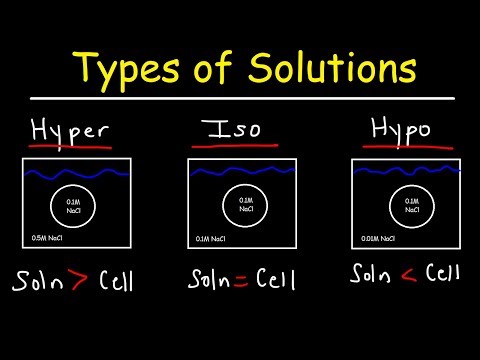
The ability of an extracellular solution to make water move into or out of a cell by osmosis is know as its tonicity. A solution's tonicity is related to its osmolarity, which is the total concentration of all solutes in the solution.
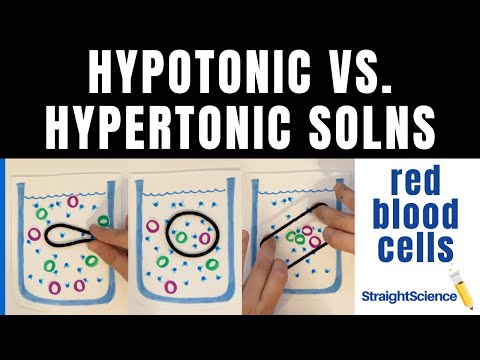
In the case of a red blood cell, isotonic conditions are ideal, and your body has homeostatic (stability-maintaining) systems to ensure these conditions stay constant. If placed in a hypotonic solution, a red blood cell will bloat up and may explode, while in a hypertonic solution, it will shrivel—making the cytoplasm dense and its contents concentrated—and may die.
Water moves readily across cell membranes through special protein-lined channels, and if the total concentration of all dissolved solutes is not equal on both sides, there will be net movement of water molecules into or out of the cell. Whether there is net movement of water into or out of the cell and which direction it moves depends on whether the cell’s environment is isotonic, hypotonic, or hypertonic.
Комментарии
 0:04:46
0:04:46
 0:02:52
0:02:52
 0:02:23
0:02:23
 0:06:29
0:06:29
 0:25:45
0:25:45
 0:04:36
0:04:36
 0:01:00
0:01:00
 0:04:28
0:04:28
 0:45:48
0:45:48
 0:16:26
0:16:26
 0:07:36
0:07:36
 0:11:55
0:11:55
 0:09:20
0:09:20
 0:09:14
0:09:14
 0:13:09
0:13:09
 0:05:04
0:05:04
 0:02:24
0:02:24
 0:01:32
0:01:32
 0:06:30
0:06:30
 0:07:07
0:07:07
 0:07:55
0:07:55
 0:02:19
0:02:19
 0:04:08
0:04:08
 0:14:34
0:14:34