filmov
tv
Exothermic and endothermic dissolution | Solubility | Chemistry

Показать описание
Dissolution of substances in water sometimes involves exchange of heat. In this video 3 compounds sodium hydroxide, ammonium nitrate and sodium chloride are dissolved in water. The temperatures of the solutions are noted after each dissolution. The sodium hydroxide dissolves with release of heat making it an exothermic dissolution. The ammonium nitrate dissolves by absorbing heat; an endothermic dissolution. Only the sodium chloride dissolves with a marginal change of temperature.
Exothermic and endothermic dissolution | Solubility | Chemistry
What Are Endothermic & Exothermic Reactions | Chemistry | FuseSchool
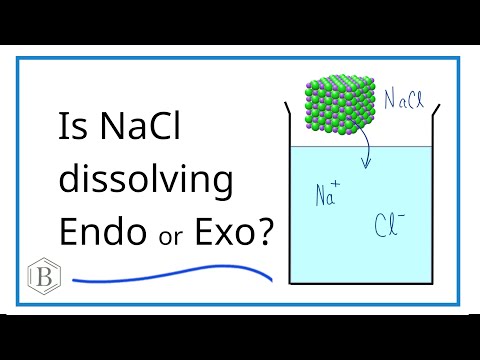
Is NaCl Dissolving Endothermic or Exothermic?
GCSE Chemistry - Exothermic and Endothermic Reactions
endothermic dissolution of ammonium nitrate
Endothermic and Exothermic Dissolution Reactions
Temperature Changes in Dissolving
Exothermic and Endothermic Processes, Formation of Solution, Thermodynamics - Chemistry
W#23 Exothermic and endothermic dissolutions
Endothermic and Exothermic Dissolution
SOL 6: Endothermic and Exothermic Dissolution
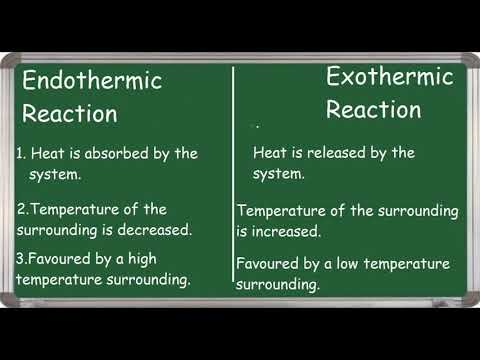
Endothermic Reaction and Exothermic Reaction
exothermic dissolution of calcium chloride
Endothermic and Exothermic Changes| Energy Changes| Introduction
Exothermic and Endothermic Reaction🧪 | Explained by Pankaj Sir #physicsexplained #pankajsirclasses...
Understanding Endothermic and Exothermic Reaction 🧪 Subscribe for more #ScienceExperiments
Endothermic reaction of adding salt to water
31 Endothermic and Exothermic Processes - Solutions
DoR#1 Exothermic and Endothermic Reactions
Energy Changes︱Exothermic and Endothermic
Endothermic Vs Exothermic reaction differences
Enthalpy of Solution, Enthalpy of Hydration, Lattice Energy and Heat of Formation - Chemistry
CS What is the difference between exothermic and endothermic reactions
Chapter Solution||Heat of Solution||Endothermic & Endothermic Process||With Examples
Комментарии
 0:02:41
0:02:41
 0:04:17
0:04:17
 0:02:25
0:02:25
 0:05:21
0:05:21
 0:00:53
0:00:53
 0:01:16
0:01:16
 0:05:24
0:05:24
 0:05:28
0:05:28
 0:07:25
0:07:25
 0:03:48
0:03:48
 0:06:34
0:06:34
 0:10:45
0:10:45
 0:00:53
0:00:53
 0:04:08
0:04:08
 0:00:54
0:00:54
 0:00:36
0:00:36
 0:01:38
0:01:38
 0:03:08
0:03:08
 0:06:16
0:06:16
 0:04:26
0:04:26
 0:06:35
0:06:35
 0:16:40
0:16:40
 0:00:16
0:00:16
 0:17:58
0:17:58