filmov
tv
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (4 of 23) State of a System

Показать описание
In this video I will give and explain the state of a system is uniquely defined by the 3 state variables P, T, V; and an adiabatic wall, diathermal wall, process, cyclical process, and quasi static process.
Next video in this series can be seen at:
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (1 of 25) Basic Terms
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (22 of 25) PVT Surfaces for Real Substances
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (16 of 25) The van der Waals Constant
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (23 of 25) PT Diagrams
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (24 of 25) Triple Point T and T
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (4 of 23) State of a System
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (2 of 25) Basic Terms and Properties
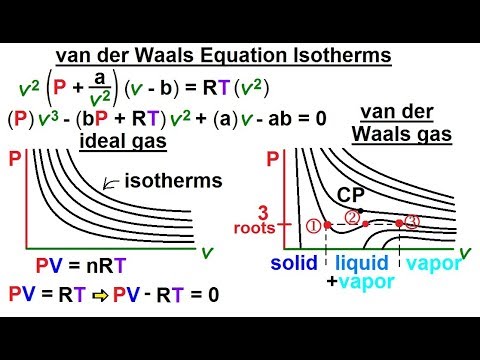
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (20 of 25) van der Waals Equation Isotherms
Thermodynamics 🔥👉🏻PYQ 2️⃣| NEET | Chemistry | Physics | Tamil #anfaz#neettamil#neet2025#neetphysics...
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (3 of 23) Molecule Density and Quantity
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (25 of 25) Critical Point Values
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (14 of 25) The PVT Surface
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (15 of 25) The van der Waals Equation
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (13 of 23) Avogadro's Law***
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (10 of 23) Boyle's Law
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (8 of 23) The Ideal Gas Equation
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (18 of 25) The van der Waals Eqn Interperted 2
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (9 of 23) What is the Gas Constant?
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (17 of 25) The van der Waals Eqn Interperted 1
Physics - Thermodynamics 2: Ch 32.2 PVT Partial Derivatives (1 of 23) The PVT Surface
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (21 of 25) van der Waals Eqn Isotherms Other Form
Physics - Thermodynamics 2: Ch 32.2 PVT Partial Derivatives (2 of 23) Keeping Pressure Constant
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (5 of 23) Thermodynamic Precesses
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (11 of 23) Guy-Lussac's Law
Комментарии
 0:04:46
0:04:46
 0:04:07
0:04:07
 0:05:55
0:05:55
 0:07:01
0:07:01
 0:03:24
0:03:24
 0:06:11
0:06:11
 0:04:30
0:04:30
 0:03:56
0:03:56
 1:12:34
1:12:34
 0:03:35
0:03:35
 0:04:21
0:04:21
 0:03:34
0:03:34
 0:07:02
0:07:02
 0:02:50
0:02:50
 0:04:23
0:04:23
 0:03:33
0:03:33
 0:02:53
0:02:53
 0:04:06
0:04:06
 0:04:24
0:04:24
 0:03:11
0:03:11
 0:05:34
0:05:34
 0:04:09
0:04:09
 0:03:44
0:03:44
 0:03:39
0:03:39