filmov
tv
Astronomy - Ch. 9.1: Earth's Atmosphere (51 of 61) The CO2 and H2O Overlap Spectrum

Показать описание
In this video I will compare the absorption spectrum of CO2 with the absorption spectrum of H2O and its transmission window at various wavelengths for the radiated energy from the Earth's surface.
Next video in this series can be seen at:
Astronomy - Ch. 9.1: Earth's Atmosphere (1 of 61) Atmospheric Content
Astronomy - Ch. 9: Earth as a Planet (1 of 22) Earth's Place in the Solar System
Astronomy - Ch. 9.1: Earth's Atmosphere (9 of 61) How a Gas Molecule Absorbs Radiation
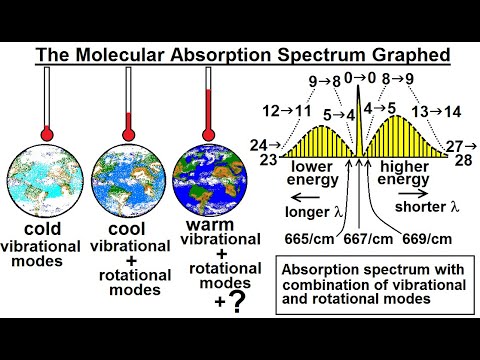
Astronomy - Ch. 9.1: Earth's Atmosphere (15 of 61) The Molecular Absorption Spectrum Graphed
Astronomy - Ch. 9.1: Earth's Atmosphere (10 of 61) 3 Types of Energy of a Gas Molecule
Astronomy - Ch. 9.1: Earth's Atmosphere (16 of 61) The Key to Our Survival: Spectrum Broadening
Astronomy - Ch. 9.1: Earth's Atmosphere (4 of 61) What is the Greenhouse Effect?
Astronomy - Ch. 9.1: Earth's Atmosphere (61 of 61) The Greenhouse Effect: What can we Conclude?
Motion | Class 9 Physics | NCERT Chapter 8 Explanation | EduCartoon Academy
Astronomy - Ch. 9.1: Earth's Atmosphere (29 of 61) The Daily Warming Cycle: Part 1
Astronomy - Ch. 9.1: Earth's Atmosphere(52 of 61) What is the G.H. Effect Contribution of CO2?(...
Astronomy - Ch. 9.1: Earth's Atmosphere (14 of 61) What Causes the Wide Absorption Spectrum?
Astronomy - Ch. 9.1: Earth's Atmosphere (39 of 61) How Does Increasing CH4 Change G.H. Effect?
Astronomy - Ch. 9.1: Earth's Atmosphere (37 of 61) The Jet Stream Effect
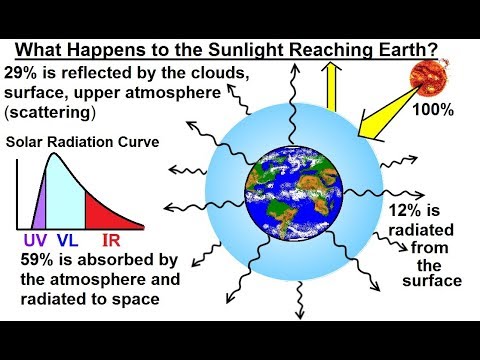
Astronomy - Ch. 9.1: Earth's Atmosphere (3 of 61) What Happens to Sunlight when it Reaches eart...
Astronomy - Ch. 9.1: Earth's Atmosphere (57 of 61) Is Atmospheric Water Vapor Increasing?
Astronomy - Ch. 9.1: Earth's Atmosphere (31 of 61) The Daily Warming Cycle: Part 3
Astronomy - Ch. 9.1: Earth's Atmosphere (25 of 46) What is the Greenhouse Effect?
Astronomy - Ch. 9.1: Earth's Atmosphere (51 of 61) The CO2 and H2O Overlap Spectrum
Astronomy - Ch. 9.1: Earth's Atmosphere (44 of 61) The Atmospheric Effectiveness of CO2? Part 2
Astronomy - Ch. 9.1: Earth's Atmosphere (24 of 61) How is the Lower Atmosphere Heated? (Part 2)
Astronomy - Ch. 9.1: Earth's Atmosphere (17 of 61) How Does CO2 Keep Us Warm?
Astronomy - Ch. 9: Earth as a Planet (13 of 22) Earth's Atmosphere: 1
Astronomy - Ch. 9.1: Earth's Atmosphere (38 of 61) The Movement of Large Air Masses
Комментарии
 0:07:35
0:07:35
 0:05:02
0:05:02
 0:06:07
0:06:07
 0:05:10
0:05:10
 0:05:34
0:05:34
 0:06:24
0:06:24
 0:05:59
0:05:59
 0:05:07
0:05:07
 0:16:11
0:16:11
 0:04:47
0:04:47
 0:04:15
0:04:15
 0:04:09
0:04:09
 0:04:35
0:04:35
 0:03:42
0:03:42
 0:05:50
0:05:50
 0:03:34
0:03:34
 0:04:03
0:04:03
 0:08:35
0:08:35
 0:02:02
0:02:02
 0:02:31
0:02:31
 0:04:57
0:04:57
 0:09:03
0:09:03
 0:08:39
0:08:39
 0:03:26
0:03:26