filmov
tv
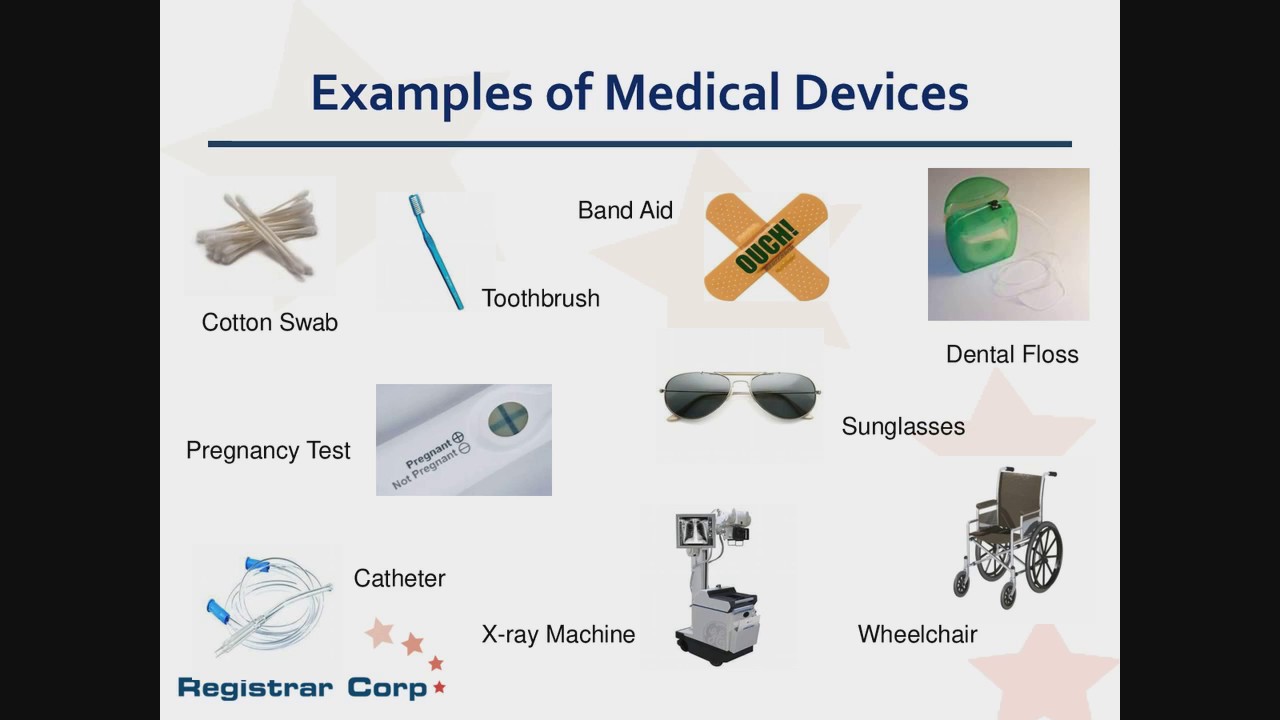
FDA 101 for Medical Devices
Показать описание
Registrar Corp's webinar provides industry with important information regarding U.S. FDA regulation of medical devices, including:
- Annual Registration Renewal
- eMDR Requirements for Adverse Events
- Unique Device Identifier (UDI) Requirements
- Annual Registration Renewal
- eMDR Requirements for Adverse Events
- Unique Device Identifier (UDI) Requirements
FDA 101 for Medical Devices
Medical Devices 101: An Entry Level Overview of the FDA
Risk Basics for Medical Devices
Medical Device 101 - Class I, II, III & De Novo Explained
FDA 101
How is My Medical Device Classified?
CTSI Watch and Learn: FDA 101: A Primer on IDEs
Understanding the FDA Medical Device 510k Process
145 - Updated Tools for Medical Device Development from FDA's Research Division
Case Study: How is My Medical Device Classified?
Design Control for Medical Devices - Online introductory course
Is the FDA Too Tough on Medical Device Makers?
Medical device regulation (FDA)
Medical Device Regulations / FDA Approval
Medical Device Regulation Primer: Focus on Digital Health - Medventions Lecture Series
Medical Device Registration in the US- An Overview of Pathways and Classification
Introducing our New FDA Class I Medical Devices
FDA's MDEpiNet - Improving Medical Device Safety and Effectiveness
Tips for Clinicians - Keeping Your Patients’ Connected Medical Devices Safe
Idea to IDE: A Medical Device in the Making
FDA Regulatory Education for Industry (REdI) – Devices and Biologics Track
Understanding the Power of the FDA Pre-Sub: Tips for a Successful Meeting
FDA looks into the risk of medical devices being hacked
Navigating the FDA Medical Device Classification Process
Комментарии
 0:57:47
0:57:47
 0:49:08
0:49:08
 0:23:18
0:23:18
 0:03:50
0:03:50
 0:19:34
0:19:34
 0:16:18
0:16:18
 0:08:23
0:08:23
 0:02:19
0:02:19
 0:42:50
0:42:50
 0:17:43
0:17:43
 0:17:31
0:17:31
 0:05:13
0:05:13
 0:54:15
0:54:15
 0:09:28
0:09:28
 1:28:01
1:28:01
 0:04:46
0:04:46
 0:00:45
0:00:45
 0:03:59
0:03:59
 0:03:13
0:03:13
 0:10:29
0:10:29
 8:58:15
8:58:15
 0:59:28
0:59:28
 0:08:40
0:08:40
 0:04:23
0:04:23