filmov
tv
Calculate the ΔH°rxn for the following reaction.

Показать описание
Calculate the ΔH°rxn for the following reaction.
ΔH°f [AsH3(g)] = 66.4 kJ/mol
ΔH°f [H3AsO4(aq)] = -904.6 kJ/mol
ΔH°f [H2O(l)] = -285.8 kJ/mol
H3AsO4(aq) + 4H2(g) → AsH3(g) + 4H2O(l)
ΔH°f [AsH3(g)] = 66.4 kJ/mol
ΔH°f [H3AsO4(aq)] = -904.6 kJ/mol
ΔH°f [H2O(l)] = -285.8 kJ/mol
H3AsO4(aq) + 4H2(g) → AsH3(g) + 4H2O(l)
 0:04:58
0:04:58
 0:14:03
0:14:03
 0:02:00
0:02:00
 0:11:39
0:11:39
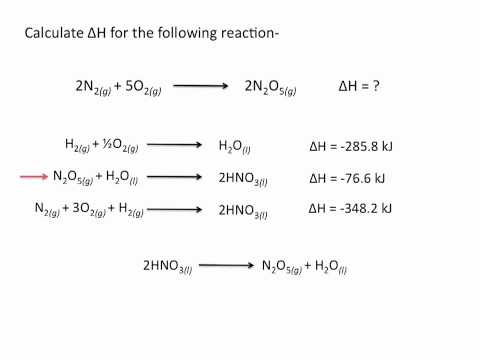 0:11:23
0:11:23
![[Example] How to](https://i.ytimg.com/vi/nmNQUGt6NiM/hqdefault.jpg) 0:01:22
0:01:22
 0:05:29
0:05:29
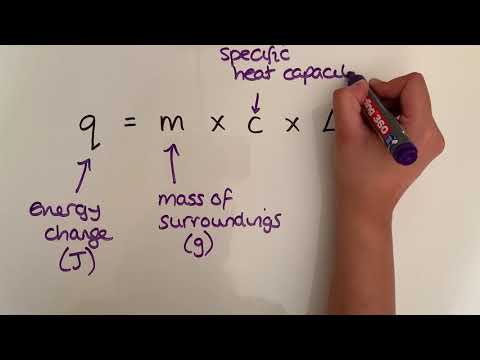 0:07:22
0:07:22
 0:05:11
0:05:11
 0:03:50
0:03:50
 0:06:29
0:06:29
 0:06:41
0:06:41
 0:00:48
0:00:48
 0:04:17
0:04:17
 0:07:49
0:07:49
 0:02:48
0:02:48
 0:07:22
0:07:22
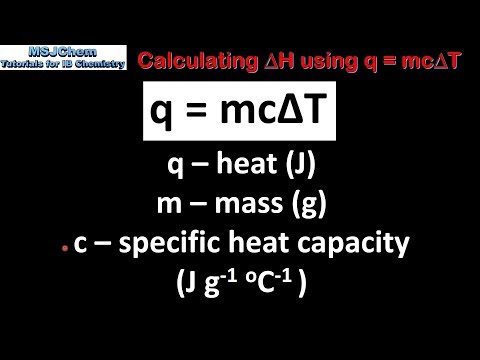 0:03:35
0:03:35
 0:03:11
0:03:11
 0:08:24
0:08:24
 0:16:42
0:16:42
 0:05:31
0:05:31
 0:06:39
0:06:39
 0:00:48
0:00:48