filmov
tv
1 mole of Iron reacts completely with 0.65 moles of oxygen to give mixture of only FeO and Fe2O3.

Показать описание
#NEET #JEE
1 mole of Iron reacts completely with 0.65 moles of oxygen to give mixture of only FeO and Fe2O3.
1.0 mole of Fe reacts completely with 0.65 mole of `O_(2)` to give a mixture of only FeO and
One mole of iron [55.8gm], when oxidised to +2 oxidation states gives up:
One mole on iron [55.8 gm], when oxidised to `+2` oxidation state gives up :
weight of oxygen in 1 mole each Fe2O3 and FeO is in the simple ratio of
9 1 Mole relationships in Chemical Equations
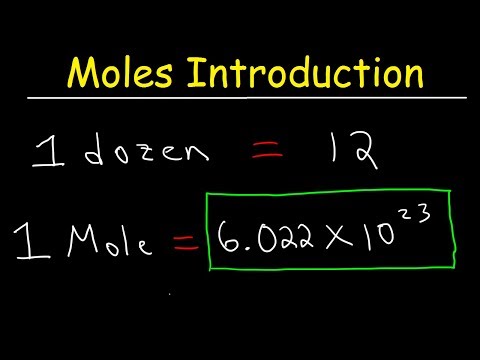
Introduction to Moles
How many moles are in 27.0 g of H2O ?
Redox Reaction Worked Example 1
Calculating masses in reactions - p27 (Chem)
2021 P1 Q25 - Redox Reaction between Chromite and Oxygen
5.58 | How many kilojoules of heat will be released when exactly 1 mole of iron, Fe, is burned to
One mole of iron, when oxidised to +2 oxidation state gives up:....
(5.7) Mole Relationships in Chemical Equations
How to calculate number of moles|| chemistry
For the reaction \( \mathrm{Fe}_{2} \mathrm{O}_{3}+3 \mathrm{CO}_{2} \rightarrow 2 \mathrm{Fe}+3...
One mole mixture of FeO and `Fe_(3)O_(4)` containing equal moles of each on reaction with excess
Balance: Fe + O2 = Fe2O3
When the supply of oxygen is limited, iron metal reacts with oxygen to produce a mixture of and In a
Rare Elements #shorts #bluebox
Stoichiometry Part 1 Mole Ratios and Road Maps
The reaction when one mole of FeC2O4 is oxidised by how many no of mole KMnO4 in an acidic medium?
Calculate Amount of Reactant Needed (Example)
RUSTING OF IRON - Chemical Reactions | SPM
Комментарии
 0:05:00
0:05:00
 0:03:20
0:03:20
 0:01:02
0:01:02
 0:01:43
0:01:43
 0:01:31
0:01:31
 0:06:24
0:06:24
 0:05:16
0:05:16
 0:03:14
0:03:14
 0:12:43
0:12:43
 0:05:54
0:05:54
 0:15:42
0:15:42
 0:08:58
0:08:58
 0:02:33
0:02:33
 0:19:15
0:19:15
 0:03:16
0:03:16
 0:02:46
0:02:46
 0:05:35
0:05:35
 0:01:39
0:01:39
 0:05:58
0:05:58
 0:00:41
0:00:41
 0:12:09
0:12:09
 0:04:18
0:04:18
 0:03:42
0:03:42
 0:10:41
0:10:41