filmov
tv
Difference between Atoms and Elements
Показать описание
The Periodic Table provides a list of all of the known Elements. In this video we’ll look at the difference between the term elements and atoms.
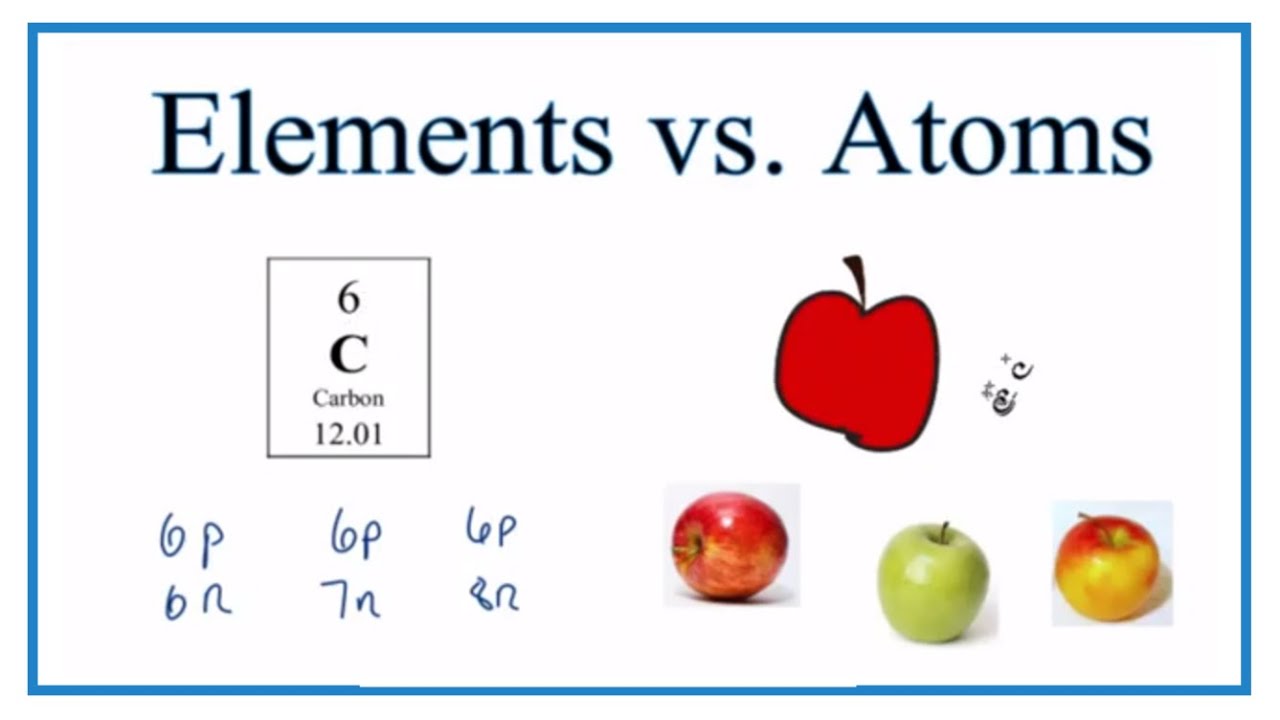
An atom is an individual particle made up of protons, neutrons, and electrons. We could say “the element Carbon is made up of atoms with six protons and either six, seven, or eight neutrons. ”
Atoms that have the same atomic number are considered to be the same element. For example, Carbon (C), Gold (Au), or Hydrogen (H). We say “element” we are referring to a category of atoms with the same number of protons. The number of neutrons may differ. We call these isotopes of that element.
Help Videos:
Images:
An atom is an individual particle made up of protons, neutrons, and electrons. We could say “the element Carbon is made up of atoms with six protons and either six, seven, or eight neutrons. ”
Atoms that have the same atomic number are considered to be the same element. For example, Carbon (C), Gold (Au), or Hydrogen (H). We say “element” we are referring to a category of atoms with the same number of protons. The number of neutrons may differ. We call these isotopes of that element.
Help Videos:
Images:
Difference between Atoms and Elements
What is the difference between an Atom, Element, Molecule and Compound?
Difference between an Atom, a Molecule and a Compound
Atom vs Element: Differences Explained
Elements, Atoms, Molecules, Ions, Ionic and Molecular Compounds, Cations vs Anions, Chemistry
Types of Matter: Elements, Compounds, and Mixtures
Difference between Atom, Element, and Molecule | Easy trick with Animation I Basic Chemistry #shorts
Elements and atoms | Atoms, compounds, and ions | Chemistry | Khan Academy
Atoms and Molecules: Revision Session for Class 9 Students | Class 9 Chemistry | LIVE
What is the difference between Atom, Element, Compound and Molecule? Basic concepts of chemistry
What is difference between atoms, ions, molecules, elements and compounds??❓🙆♂️🙆♂️...
What Distinguishes Compounds from Molecules?
Atoms, Elements, and Molecules
What are Atoms? The smallest parts of Elements and YOU!
What are the differences between Atoms and Elements - Chemistry Revision (Years 7, 8 & 9)
How Small Is An Atom? Spoiler: Very Small.
Chemistry with LEGOS - Atoms & Elements Explained - For Kids!
What is an atom | Matter | Physics | FuseSchool
Atoms for Kids | What is an atom? | Learn about atoms and molecules with activities and worksheets
What's Inside an Atom? Protons, Electrons, and Neutrons!
Atom Explained in Simple Terms
Difference between Atoms and Ions (Explanation & Examples)
How atoms bond - George Zaidan and Charles Morton
How small are atoms?
Комментарии
 0:04:19
0:04:19
 0:06:31
0:06:31
 0:02:12
0:02:12
 0:01:59
0:01:59
 0:13:53
0:13:53
 0:04:15
0:04:15
 0:00:50
0:00:50
 0:13:09
0:13:09
 0:37:26
0:37:26
 0:04:47
0:04:47
 0:00:58
0:00:58
 0:03:49
0:03:49
 0:03:56
0:03:56
 0:04:23
0:04:23
 0:05:21
0:05:21
 0:04:58
0:04:58
 0:03:47
0:03:47
 0:04:00
0:04:00
 0:06:45
0:06:45
 0:04:06
0:04:06
 0:01:44
0:01:44
 0:07:30
0:07:30
 0:03:34
0:03:34
 0:00:48
0:00:48