filmov
tv
The 18 Electron Rule for Transition Metal Complexes
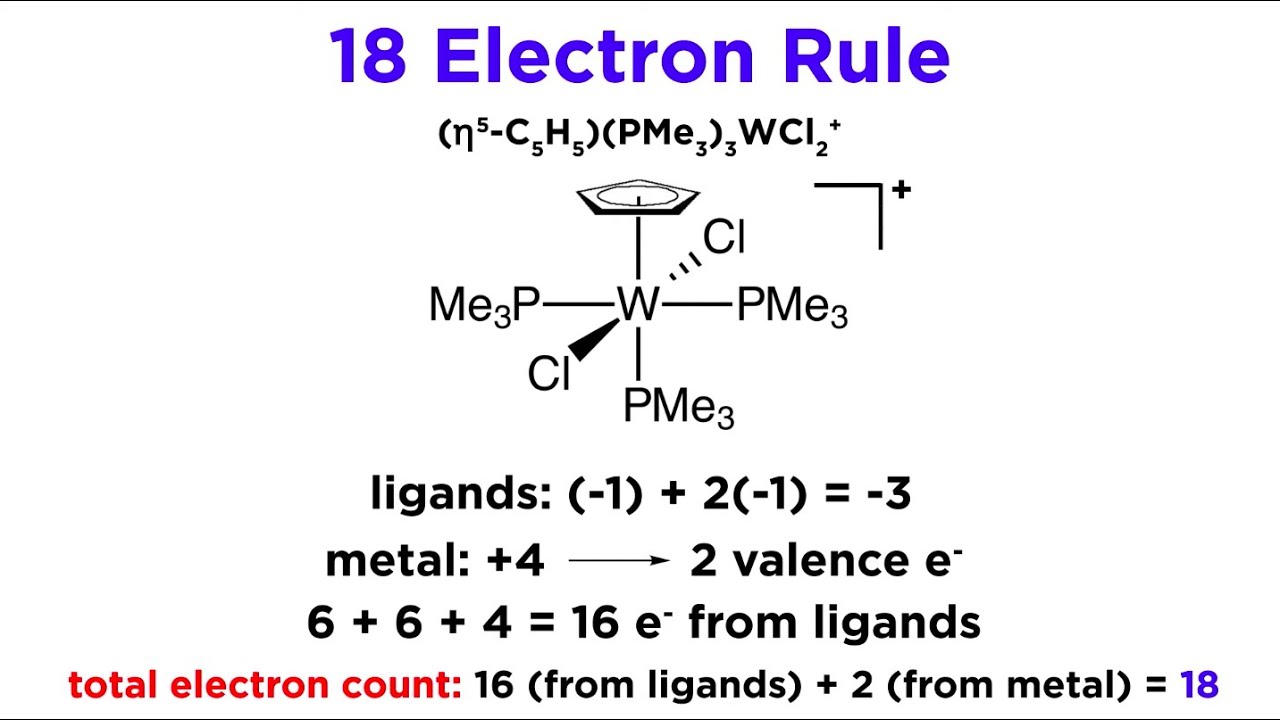
Показать описание
Ok, so we understand how ligands bond to metals to form transition metal complexes, but how many ligands will fit? Well, remember the octet rule from general chemistry? There is a similar concept that we can apply here, which is called the 18 electron rule. 18 is bigger than 8 because transition metals have access to d orbitals. Let's see how this rule works now!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
The 18 Electron Rule for Transition Metal Complexes
More Practice With the 18 Electron Rule
18-electron rule
7.8. The 18 Electron Rule
18 Electron Rule - Electron Counting in Coordination Chemistry | Professor Adam Teaches
18 Electron Rule - Definition, Electron counting common ligands and Examples
Organometallic Chemistry Basics I: The 18 Electron Rule
18 electron rule for organometallic compounds|Organometallic chemistry|inorganic chemistry CSIR-NET
special trick about Electronic configuration.s.p.d.f rule.
18 electron rule with example | organometallic chemistry
Polynuclear Transition Metal Complexes
18 Electron Rule | Stability of Coordination Complexes | For CSIR NET
The 18 Electron Rule | Naming complexes
What are the limitations of the 18-electron rule?
18 electron rule .(Organometallic compound) part 1
What is the exception of 18 electron rule?
18 Electron Rule Key points, Examples, 16 Electron Rule, Exceptions
18 Electron Rule (Solved Examples) for Organometallic Compounds, Organometallic Chemistry, Lec-02
18 Electron Rule (Previous Years Solved Problems Part-A) Lec-03 for Organometallic Compounds
18 electron rule for ORGANOMETALLIC COMPOUNDS and its LIMITATIONS. NET&GATE
Examples of electron counts in organometallic complexes
EAN | Effective Atomic Number | 18 electron rule
EAN & 18 electron rules for metal carbonyls bsc, msc chemistry
18 Electron Rule for Organometallic Compounds, Organometallic Chemistry, Inorganic Chemistry
Комментарии
 0:10:45
0:10:45
 0:06:14
0:06:14
 0:06:20
0:06:20
 0:08:15
0:08:15
 0:12:45
0:12:45
 0:18:51
0:18:51
 0:19:48
0:19:48
 0:39:19
0:39:19
 0:08:17
0:08:17
 0:08:54
0:08:54
 0:07:38
0:07:38
 0:22:26
0:22:26
 1:13:24
1:13:24
 0:00:47
0:00:47
 0:05:11
0:05:11
 0:00:48
0:00:48
 0:25:08
0:25:08
 0:39:10
0:39:10
 0:19:48
0:19:48
 0:07:54
0:07:54
 0:11:32
0:11:32
 0:07:59
0:07:59
 0:22:43
0:22:43
 0:39:37
0:39:37