filmov
tv
Production of Ammonia (NH3) gas in Lab | Board Exam Experiment | Grade 10 | Chemistry

Показать описание
Chemical Reaction :-
NH4Cl + NaOH = NaCl + NH3 + H2O
Properties of Ammonia Gas:-
1. Pungent smelling gas capable of bringing tears into your eyes.
2. It is basic in in nature, when dissolved in water forms ammonium
Hydroxide which is a base.
Test For ammonia gas
1. As ammonia gas is basic in nature it turns red litmus paper blue.
2. When a glass rod dipped in conc. HCl is brought near to the mouth of the test tube containing NH3 gas, dense white fumes of ammonium chloride is formed.
NH4Cl + NaOH = NaCl + NH3 + H2O
Properties of Ammonia Gas:-
1. Pungent smelling gas capable of bringing tears into your eyes.
2. It is basic in in nature, when dissolved in water forms ammonium
Hydroxide which is a base.
Test For ammonia gas
1. As ammonia gas is basic in nature it turns red litmus paper blue.
2. When a glass rod dipped in conc. HCl is brought near to the mouth of the test tube containing NH3 gas, dense white fumes of ammonium chloride is formed.
What Is The Haber Process | Reactions | Chemistry | FuseSchool
Ammonia—a renewable fuel made from sun, air, and water—could power the globe without carbon
What is green ammonia?
Manufacture process of Ammonia (NH3) | Chemical engineering
NH3 | Ammonia | Sources and Impact| OIZOM Academy
Production of Ammonia (NH3) gas in Lab | Board Exam Experiment | Grade 10 | Chemistry
How to transport hydrogen using ammonia (NH3)
Chemistry - Haber Process for the production of Ammonia NH3
Make Concentrated Ammonia
Production of Nitrogen Fertilizer
The Haber Process - the Uses of Ammonia | Reactions | Chemistry | FuseSchool
Ammonia (NH3) production (Video sample) #ChemicalEngineering #CBAI
Ammonia: The Next Big Thing in Energy Production - GenCell
Uses Of Ammonia Gas (NH3) | Applications
One of the largest single-train ammonia plants worldwide
Reaction of NH3 (g) + HCl (g) Can two gases make a solid? 🌪
Test for ammonia gas
Ammonia refrigeration. Easy to understand. Animation
Ammonia (NH3)
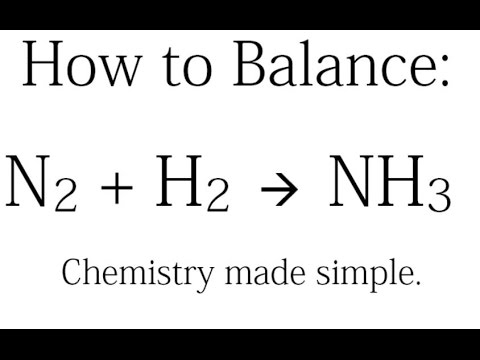
How to Balance: N2 + H2 = NH3 (Synthesis of Ammonia)
Characteristics of ammonia gas | Properties of NH3
What Is Ammonia ?
Chemistry - Chemical Kinetics (27 of 30) The Haber Synthesis of Ammonia, NH3
Ammonia NH3: 3D Animation Molecule
Комментарии
 0:04:05
0:04:05
 0:03:01
0:03:01
 0:01:08
0:01:08
 0:06:27
0:06:27
 0:03:46
0:03:46
 0:04:32
0:04:32
 0:07:04
0:07:04
 0:03:08
0:03:08
 0:10:02
0:10:02
 0:03:15
0:03:15
 0:04:24
0:04:24
 0:00:42
0:00:42
 0:02:17
0:02:17
 0:02:58
0:02:58
 0:04:38
0:04:38
 0:01:21
0:01:21
 0:01:30
0:01:30
 0:01:54
0:01:54
 0:11:52
0:11:52
 0:01:00
0:01:00
 0:01:14
0:01:14
 0:02:46
0:02:46
 0:03:04
0:03:04
 0:00:26
0:00:26