filmov
tv
Western blots: the what's, why's, and which's, of the workflow

Показать описание
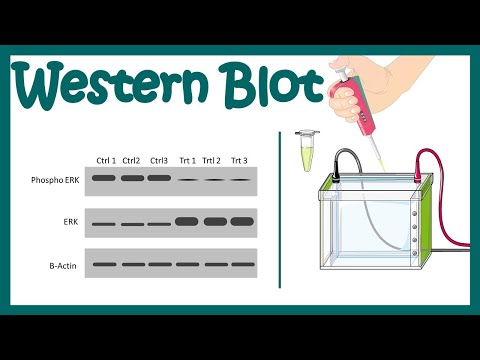
western blots… SDS-PAGE lets us separate proteins by size, but doesn’t tell us what the proteins we separated are. If you add on a second technique, called a western blot, you can use labeled antibody probes to test for the presence and quantity of proteins of interest. It won’t tell you what all the proteins in your sample are, just the ones you test for (kinda like a molecular game of Go Fish!). And you’ll have to have a lot of patience, but it’s a fundamental biochemistry technique, so let’s look at how it works.
The western blot is an experiment used to answer questions like - How much protein X does a cell make under different conditions? The basic premise is - take some mix of proteins (such as the “lysate “you get when you break open (lyse) a cell) → send them traveling vertically though a gel mesh to separate them by size → trap them in place → send them traveling horizontally out of the gel onto a membrane → use antibodies (proteins that recognize specific other proteins) to probe the membrane to see if and how much of a specific protein is there.
Here’s a general layout of the experiment:
SDS-PAGE - separate proteins by size & trap them in a gel
TRANSFER/BLOT - move the trapped proteins to a membrane that likes to bind proteins - kinda like a “protein duct tape.” The membrane doesn’t feel sticky to your fingers but it does to proteins (but not after you’ve touched it with your fingers - so use gloves and tweezers!)
BLOCK - prevent nonspecific antibody binding by getting “generic” proteins to bind the parts of the membrane where your protein isn’t before the antibody has a chance to. Your protein’s only at small portions of the protein duct tape, so you need to coat the rest of that sticky membrane with something “generic” like bovine serum albumin (BSA) or milk (really! it’s chock full of proteins)
WASH, WASH, WASH - wash off non-bound protein
BIND PRIMARY ANTIBODY - Antibodies are little proteins that recognize & bind specifically to specific parts of other things, with the “other thing” being called an ANTIGEN and the “specific parts” being EPITOPES. In a western blot, the antigen is a protein you’re looking for and the epitope is a specific site on that protein. The primary antibody recognizes your specific protein and binds it, so you get your specificity for the thing you’re looking for - yay! but it doesn’t have anything “seeable” about it - boo 😢
WASH, WASH, WASH - wash off non-bound primary antibody
BIND SECONDARY ANTIBODY - this recognizes your primary antibody and has some “detectable” quality like a fluorophore so it will emit light or an enzyme like horse radish peroxidase (HRP) that will convert an uncolored compound you add to a colored compound (chromogenic method) or light (chemiluminescence). It works because antibodies have a unique part that recognizes some antigen and a “generic adapter part” - but that generic adapter part’s only generic for the particular animal that made it (i.e. the adapter part’s slightly different in mice & rats). So you can use things like “goat anti-rat” which is a secondary antibody that uses its unique part to recognize the generic adapter part of a primary antibody made by a rat (and if that rat antibody is using its unique part to bind your protein…)
WASH, WASH, WASH - wash off non-bound secondary antibody
VISUALIZE - detect the detectable using the detectable’s detection method
Some more details:
continued in comments
The western blot is an experiment used to answer questions like - How much protein X does a cell make under different conditions? The basic premise is - take some mix of proteins (such as the “lysate “you get when you break open (lyse) a cell) → send them traveling vertically though a gel mesh to separate them by size → trap them in place → send them traveling horizontally out of the gel onto a membrane → use antibodies (proteins that recognize specific other proteins) to probe the membrane to see if and how much of a specific protein is there.
Here’s a general layout of the experiment:
SDS-PAGE - separate proteins by size & trap them in a gel
TRANSFER/BLOT - move the trapped proteins to a membrane that likes to bind proteins - kinda like a “protein duct tape.” The membrane doesn’t feel sticky to your fingers but it does to proteins (but not after you’ve touched it with your fingers - so use gloves and tweezers!)
BLOCK - prevent nonspecific antibody binding by getting “generic” proteins to bind the parts of the membrane where your protein isn’t before the antibody has a chance to. Your protein’s only at small portions of the protein duct tape, so you need to coat the rest of that sticky membrane with something “generic” like bovine serum albumin (BSA) or milk (really! it’s chock full of proteins)
WASH, WASH, WASH - wash off non-bound protein
BIND PRIMARY ANTIBODY - Antibodies are little proteins that recognize & bind specifically to specific parts of other things, with the “other thing” being called an ANTIGEN and the “specific parts” being EPITOPES. In a western blot, the antigen is a protein you’re looking for and the epitope is a specific site on that protein. The primary antibody recognizes your specific protein and binds it, so you get your specificity for the thing you’re looking for - yay! but it doesn’t have anything “seeable” about it - boo 😢
WASH, WASH, WASH - wash off non-bound primary antibody
BIND SECONDARY ANTIBODY - this recognizes your primary antibody and has some “detectable” quality like a fluorophore so it will emit light or an enzyme like horse radish peroxidase (HRP) that will convert an uncolored compound you add to a colored compound (chromogenic method) or light (chemiluminescence). It works because antibodies have a unique part that recognizes some antigen and a “generic adapter part” - but that generic adapter part’s only generic for the particular animal that made it (i.e. the adapter part’s slightly different in mice & rats). So you can use things like “goat anti-rat” which is a secondary antibody that uses its unique part to recognize the generic adapter part of a primary antibody made by a rat (and if that rat antibody is using its unique part to bind your protein…)
WASH, WASH, WASH - wash off non-bound secondary antibody
VISUALIZE - detect the detectable using the detectable’s detection method
Some more details:
continued in comments
Комментарии
 0:04:23
0:04:23
 0:33:36
0:33:36
 0:03:13
0:03:13
 0:12:40
0:12:40
 0:06:32
0:06:32
 0:12:34
0:12:34
 0:58:08
0:58:08
 0:02:35
0:02:35
 1:07:30
1:07:30
 0:15:23
0:15:23
 0:08:04
0:08:04
 0:45:42
0:45:42
 0:10:56
0:10:56
 0:35:56
0:35:56
 0:16:28
0:16:28
 0:09:13
0:09:13
 0:50:05
0:50:05
 0:24:42
0:24:42
 0:09:32
0:09:32
 0:00:31
0:00:31
 0:04:20
0:04:20
 0:02:34
0:02:34
 0:07:53
0:07:53
 0:00:14
0:00:14