filmov
tv
Faradays Constant and practice problems.

Показать описание
Faradays Constant and practice problems.
Faraday's Laws of Electrolysis
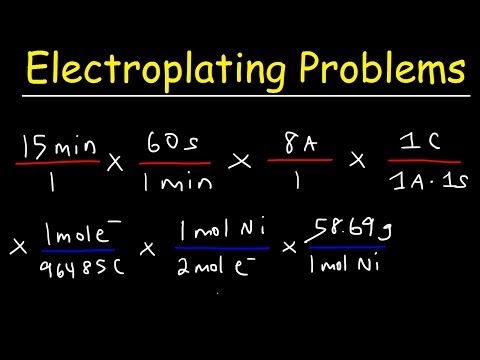
The known Faraday constant 96,485 C/mol denoted by the symbol F, or also called 1 F, corresponds to the amount of electricity that is carried by 1 mol of electrons. The number comes from the charge of a single electron 1.6023 × 10−19 C times the number of electrons in 1 mol, given by the Avogadro number 6.02 × 1023 electrons.
According to the Faraday's first law, in a given electrochemical reaction the mass (m) of substance that is deposited or released at the electrode surface is directly proportional to the amount of electricity or charge (Q) that passes through it.
In physical chemistry, the Faraday constant (symbol F, sometimes stylized as ℱ) is a physical constant defined as the quotient of the total electric charge (q) by the amount (n) of elementary charge carriers in any given sample of matter: F = q/n; it is expressed in units of coulombs per mole (C/mol). As such, it represents the "molar elementary charge",[1] i.e., the electric charge of one mole of elementary carriers (e.g., protons). It is named after the English scientist Michael Faraday. Since the 2019 redefinition of SI base units,[1] the Faraday constant has an exactly defined value, the product of the elementary charge
(4.23)m α Q or m = Z·Q
where m is the mass in grams, Q is the charge in Coulombs, and Z is the proportionality number given in g/C. The proportionality number can be translated as the electrochemical equivalent (E), which is the mass produced or consumed at the electrodes by 1 C of charge. This means that 1 C will liberate 1 g equivalent of a substance divided by 96,485, where Z can be finally expressed as Z = E/96,485. Now, if we rearrange Eq. (4.23) using Q = I·t,
Applications
One of the most common uses of Faraday constant is in electrolysis. Dividing the amount of charge in coulombs by the Faraday constant gives the amount in moles of elements that have been oxidized.
Calculation
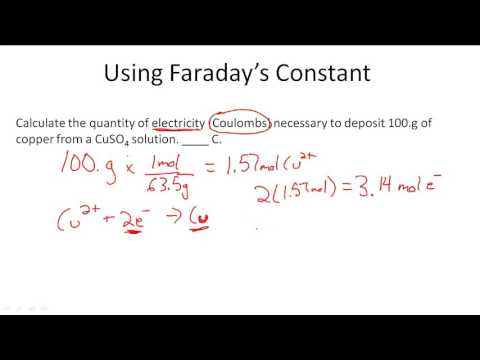
Researches have been carrying out till today to increase the accuracy of Faraday constant. Although it was initially determined using Faraday’s law of electrolysis. In general, for an electrochemical reaction, a measured value of current is made to pass for a certain time and the value of F was calculated by measuring the amount of silver deposited.
Hope you have understood about Faraday constant, How it is calculated, Its physical properties like – Value, Formula along with terms, units and values, Applications in electrolysis.
What is Faraday Constant?
It represents the magnitude of electric charge per mole of the electron.
Q2
What is the value of the Faraday Constant?
It has currently the universally accepted value
Faraday constant (F) =96485.33289(59) C mol−1
Q3
What is the formula of the Faraday Constant?
This constant can be expressed in terms of two other physical constants as
F = eNA
Where,
e is the charge of the electron in coulombs e = 1.60217662×10-19 C
NA is the Avogadro constant. NA = 6.022141×1023 mol-1.
Q4
What is the value of the Faraday Constant in kcal per volt gram equivalent?
Faraday constant (F) = 23.061 kcal per volt gram equivalent
Q5
What is the application of Faraday’s constant?
One of the most common uses of Faraday constant is in electrolysis. Dividing the amount of charge in coulombs by the Faraday constant gives the amount in moles of elements that have been oxidized.
faraday's constant ,faraday's constant electrochemistry, faraday's constant chemistry are important concepts we have to know .
faraday constant natok ,faraday constant class 12,faraday constant calculation and finally using the faraday constant musfiq faraday constant natok.
The faraday effect verdet constant is lost the constant faraday using faraday's constant aleks
0:00 - Constant derivation
2:00 - Example 1
3:30 - Example 2
#chemistry
Faraday's Laws of Electrolysis
The known Faraday constant 96,485 C/mol denoted by the symbol F, or also called 1 F, corresponds to the amount of electricity that is carried by 1 mol of electrons. The number comes from the charge of a single electron 1.6023 × 10−19 C times the number of electrons in 1 mol, given by the Avogadro number 6.02 × 1023 electrons.
According to the Faraday's first law, in a given electrochemical reaction the mass (m) of substance that is deposited or released at the electrode surface is directly proportional to the amount of electricity or charge (Q) that passes through it.
In physical chemistry, the Faraday constant (symbol F, sometimes stylized as ℱ) is a physical constant defined as the quotient of the total electric charge (q) by the amount (n) of elementary charge carriers in any given sample of matter: F = q/n; it is expressed in units of coulombs per mole (C/mol). As such, it represents the "molar elementary charge",[1] i.e., the electric charge of one mole of elementary carriers (e.g., protons). It is named after the English scientist Michael Faraday. Since the 2019 redefinition of SI base units,[1] the Faraday constant has an exactly defined value, the product of the elementary charge
(4.23)m α Q or m = Z·Q
where m is the mass in grams, Q is the charge in Coulombs, and Z is the proportionality number given in g/C. The proportionality number can be translated as the electrochemical equivalent (E), which is the mass produced or consumed at the electrodes by 1 C of charge. This means that 1 C will liberate 1 g equivalent of a substance divided by 96,485, where Z can be finally expressed as Z = E/96,485. Now, if we rearrange Eq. (4.23) using Q = I·t,
Applications
One of the most common uses of Faraday constant is in electrolysis. Dividing the amount of charge in coulombs by the Faraday constant gives the amount in moles of elements that have been oxidized.
Calculation
Researches have been carrying out till today to increase the accuracy of Faraday constant. Although it was initially determined using Faraday’s law of electrolysis. In general, for an electrochemical reaction, a measured value of current is made to pass for a certain time and the value of F was calculated by measuring the amount of silver deposited.
Hope you have understood about Faraday constant, How it is calculated, Its physical properties like – Value, Formula along with terms, units and values, Applications in electrolysis.
What is Faraday Constant?
It represents the magnitude of electric charge per mole of the electron.
Q2
What is the value of the Faraday Constant?
It has currently the universally accepted value
Faraday constant (F) =96485.33289(59) C mol−1
Q3
What is the formula of the Faraday Constant?
This constant can be expressed in terms of two other physical constants as
F = eNA
Where,
e is the charge of the electron in coulombs e = 1.60217662×10-19 C
NA is the Avogadro constant. NA = 6.022141×1023 mol-1.
Q4
What is the value of the Faraday Constant in kcal per volt gram equivalent?
Faraday constant (F) = 23.061 kcal per volt gram equivalent
Q5
What is the application of Faraday’s constant?
One of the most common uses of Faraday constant is in electrolysis. Dividing the amount of charge in coulombs by the Faraday constant gives the amount in moles of elements that have been oxidized.
faraday's constant ,faraday's constant electrochemistry, faraday's constant chemistry are important concepts we have to know .
faraday constant natok ,faraday constant class 12,faraday constant calculation and finally using the faraday constant musfiq faraday constant natok.
The faraday effect verdet constant is lost the constant faraday using faraday's constant aleks
0:00 - Constant derivation
2:00 - Example 1
3:30 - Example 2
#chemistry
Комментарии
 0:20:47
0:20:47
 0:03:20
0:03:20
 0:02:53
0:02:53
 0:08:03
0:08:03
 0:11:53
0:11:53
 0:05:55
0:05:55
 0:13:16
0:13:16
 0:53:39
0:53:39
 0:04:30
0:04:30
 0:17:42
0:17:42
 0:09:04
0:09:04
 0:09:11
0:09:11
 0:05:36
0:05:36
 0:04:45
0:04:45
 0:11:02
0:11:02
 0:13:01
0:13:01
 0:10:56
0:10:56
 0:00:53
0:00:53
 0:30:53
0:30:53
 0:02:57
0:02:57
 0:38:36
0:38:36
 0:14:23
0:14:23
 0:06:05
0:06:05
 0:09:29
0:09:29