filmov
tv
Atomic Structure FULL CHAPTER | Class 11th Physical Chemistry | Arjuna NEET

Показать описание
-------------------------------------------------------
Struggling with Atomic Structure in Chemistry for your Class 11th NEET preparation? In this comprehensive one-shot video, we've got you covered with all the essentials you need to know about the Atomic Structure in Chemistry for your NEET Preparation. Unravel the mysteries, as we break down complex concepts into simple, easy-to-understand explanations. Whether you're a beginner or looking for a quick revision before the exam, this video will boost your confidence and help you grasp the Atomic Structure in no time.
-------------------------------------------------------
Arjuna NEET - Class 11th NEET One Shot Batch Details :
- The complete syllabus of the 11th + NEET
- We will cover Physics, Chemistry, Botany, and Zoology
- All students can clear their BACKLOG through this series
- All lectures will be provided in Recorded Form
- 6 Days scheduled classes with ONE lecture per day
- PDF Notes of each Class will be uploaded on PW App
- Each lecture will be 4-5 Hours
- Classes will be starting from 02nd August 2023
-------------------------------------------------------
📌 ARJUNA NEET SOCIAL MEDIA -
-------------------------------------------------------
📌 RECOMMENDED CHANNELS FOR YOU -
-------------------------------------------------------
📌 PHYSICS WALLAH SOCIAL MEDIA -
-------------------------------------------------------
⏳ Timestamps -
00:00 - Introduction
03:49 - Basic introduction
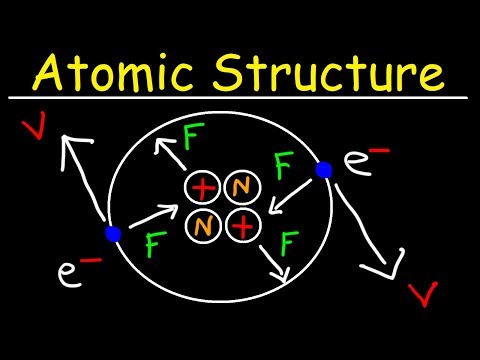
04:48 - Sub atomic particles
21:07 - Thompson's Plum Pudding model
24:32 - Alpha ray experiment / Rutherford model
52:13 - Bohr's Atomic theory
1:13:12 - Bohr atomic model
1:57:13 - Ryderberg's equation
2:16:32 - Wave particle duality / Debroglie equation
2:40:00 - Heisenberg uncertainity principle
3:11:37 - Energy of orbitals
3:27:49 - Paul's exclusion principle
3:31:32 - Aufbau's Principle
3:53:07 - Node
4:05:05 - Thank You Bacchon!
-------------------------------------------------------
-------------------------------------------------------
#NEET2024 #ArjunaNEET #AtomicStructure #Class11Chemistry#NEETPreparation #AtomicStructureInOneShot #AtomicStructureNEET
Комментарии
 0:11:45
0:11:45
 1:14:48
1:14:48
 1:28:37
1:28:37
 3:03:25
3:03:25
 0:07:02
0:07:02
 4:05:55
4:05:55
 1:21:17
1:21:17
 1:29:48
1:29:48
 0:45:56
0:45:56
 1:04:43
1:04:43
 3:27:37
3:27:37
 1:57:06
1:57:06
 7:15:12
7:15:12
 2:32:47
2:32:47
 2:32:00
2:32:00
 0:13:18
0:13:18
 0:00:22
0:00:22
 1:00:28
1:00:28
 3:32:31
3:32:31
 5:13:38
5:13:38
 7:19:15
7:19:15
 1:35:20
1:35:20
 0:05:22
0:05:22
 1:03:05
1:03:05