filmov
tv
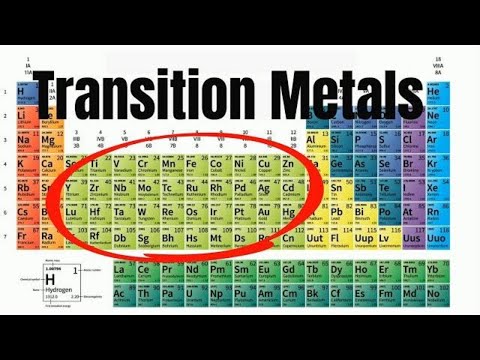
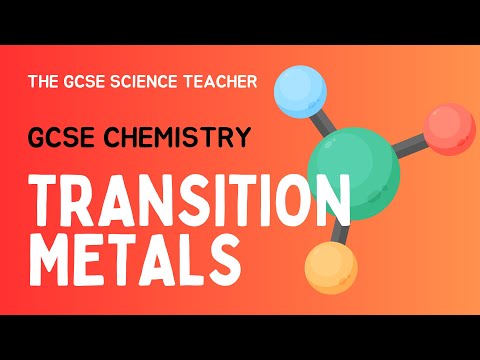
The transition metals

Показать описание
The transition metals are the elements found in groups 3-12
Technically they are element that has an incompleted subshell
Here is a list of transition metals with their number of valence electrons
The transition metals are metallic elements that serve as a bridge, or transition, between the two sides of the table. The lanthanides and the actinides at the bottom of the table are sometimes known as the inner transition metals because they have atomic numbers that fall between the first and second elements in the last two rows of the transition metals.
Transition metals tend to be very hard.
These metals look shiny and metallic. Most transition metals are grayish or white (like iron or silver), but gold and copper have other colors, not seen in any other elements on the periodic table.
The transition metals, as a group, have high melting points. The exception is mercury, which is a liquid at room temperature.
Transition elements also have high boiling points.
Although the transition metals are reactive, they are not as reactive as elements belonging to the alkali metals group.
Most transition metals have 2 electrons in their outer shell, but some have 1 electron and Palladium has 18
They all have an incompleted subshell
Thanks for stopping by MooMooMath and Science
Here is an updated version of the transition metals
What happens when you mix sodium and water?
Hint: It involves fire.
Technically they are element that has an incompleted subshell
Here is a list of transition metals with their number of valence electrons
The transition metals are metallic elements that serve as a bridge, or transition, between the two sides of the table. The lanthanides and the actinides at the bottom of the table are sometimes known as the inner transition metals because they have atomic numbers that fall between the first and second elements in the last two rows of the transition metals.
Transition metals tend to be very hard.
These metals look shiny and metallic. Most transition metals are grayish or white (like iron or silver), but gold and copper have other colors, not seen in any other elements on the periodic table.
The transition metals, as a group, have high melting points. The exception is mercury, which is a liquid at room temperature.
Transition elements also have high boiling points.
Although the transition metals are reactive, they are not as reactive as elements belonging to the alkali metals group.
Most transition metals have 2 electrons in their outer shell, but some have 1 electron and Palladium has 18
They all have an incompleted subshell
Thanks for stopping by MooMooMath and Science
Here is an updated version of the transition metals
What happens when you mix sodium and water?
Hint: It involves fire.
Комментарии
 0:03:21
0:03:21
 0:05:34
0:05:34
 0:08:12
0:08:12
 0:02:45
0:02:45
 0:01:55
0:01:55
 0:11:33
0:11:33
 0:04:45
0:04:45
 0:14:28
0:14:28
 0:05:45
0:05:45
 0:43:31
0:43:31
 0:07:30
0:07:30
 0:04:14
0:04:14
 0:05:44
0:05:44
 0:10:10
0:10:10
 0:13:33
0:13:33
 1:45:33
1:45:33
 0:13:42
0:13:42
 0:05:29
0:05:29
 0:18:10
0:18:10
 0:05:18
0:05:18
 0:07:58
0:07:58
 0:12:33
0:12:33
 0:07:21
0:07:21
 0:02:49
0:02:49