filmov
tv
Number of Lone Pairs and Bonding Pairs for BF3 (Boron trifluoride)

Показать описание
To determine the number of lone pairs and bonding pairs of electrons for BF3 we first need to draw as valid Lewis Structure. Once we have a Lewis Structure for BF3 then we can identify the lone and bonding pairs.
Bonding pairs of electrons are the electrons between the atoms. These form the chemical bond and are shared between atoms. Often a pair of bonding electrons is represented by a line. Each line represents a pair of bonding electrons.
Lone pairs of electrons are the remaining electrons around the atom. These are not between atoms and are not shared. The are important because they do occupy space and influence the shape of the molecule.
There are no lone pairs of electrons on the B atom in BF3 (Boron trifluoride).
We often need to know the the number of lone pairs of electrons in a molecule like BF3 (as well as electrons involved in bonds) to determine the molecular geometry, calculate formal charges, and understand polarity and chemical reactivity.
---Learning Resources---
Bonding pairs of electrons are the electrons between the atoms. These form the chemical bond and are shared between atoms. Often a pair of bonding electrons is represented by a line. Each line represents a pair of bonding electrons.
Lone pairs of electrons are the remaining electrons around the atom. These are not between atoms and are not shared. The are important because they do occupy space and influence the shape of the molecule.
There are no lone pairs of electrons on the B atom in BF3 (Boron trifluoride).
We often need to know the the number of lone pairs of electrons in a molecule like BF3 (as well as electrons involved in bonds) to determine the molecular geometry, calculate formal charges, and understand polarity and chemical reactivity.
---Learning Resources---
Lewis Dot Structures - How To Calculate The Number of Lone Pairs Using a Formula
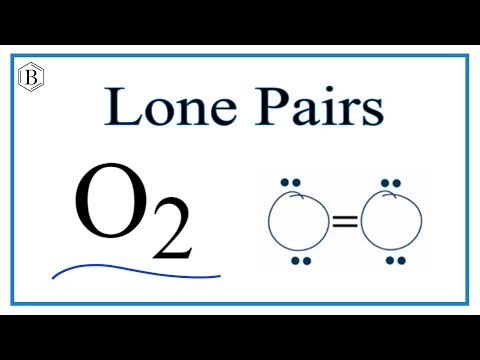
Number of Lone Pairs and Bonding Pairs for O2
How to calculate bond pair and lone pair of electrons? Easy Trick
Number of Lone Pairs and Bonding Pairs for H2O (Water)
Number of Lone Pairs and Bonding Pairs for CO
How To Identify The Number of Lone Pairs on an Atom Using Formal Charge
Number of Lone Pairs and Bonding Pairs for N2
Number of Lone Pairs and Bonding Pairs for Cl2
Basic of Organic chemistry - Chemistry - Session 14
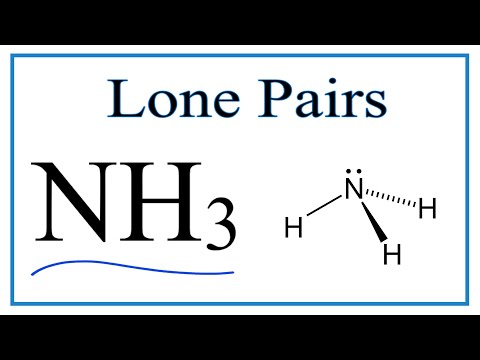
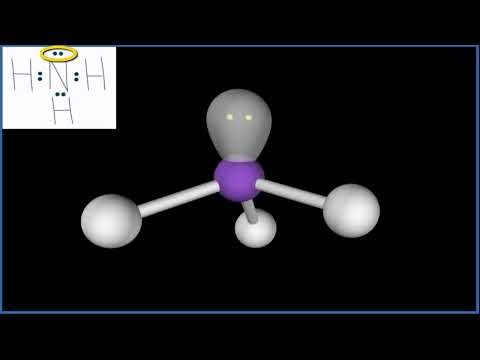
Number of Lone Pairs and Bonding Pairs for NH3 (Ammonia)
Number of Lone Pairs and Bonding Pairs for XeF4 (Xenon tetrafluoride)
Lone Pair vs Bonding Pair Electrons
Number of Lone Pairs and Bonding Pairs for BF3 (Boron trifluoride)
Number of Lone Pairs and Bonding Pairs for SO2 (Sulfur dioxide)
Number of Lone Pairs and Bonding Pairs for HCl
Lone pair and bond pair electrons | IIT JEE & NEET | Vineet Khatri | ATP STAR
Number of Lone Pairs and Bonding Pairs for NH4 +
Number of Lone Pairs and Bonding Pairs for SO3
Number of Lone Pairs and Bonding Pairs for H2S
Number of Lone Pairs and Bonding Pairs for H2
Number of Lone Pairs and Bonding Pairs for CO2 (Carbon dioxide)
Number of Lone Pairs and Bonding Pairs for NO3 - (Nitrate ion)
The Numbers of Lone Pairs and Bond Pairs are Present in Water Molecule
Trick for Hybridization & Lone Pair Find | Chemical Bonding | NEET | JEE Main & Advanced | C...
Комментарии
 0:10:09
0:10:09
 0:01:11
0:01:11
 0:06:49
0:06:49
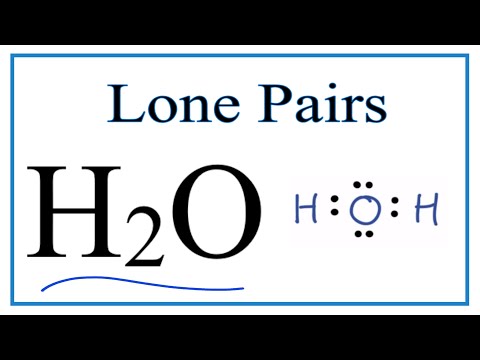 0:01:57
0:01:57
 0:01:11
0:01:11
 0:15:15
0:15:15
 0:01:09
0:01:09
 0:01:03
0:01:03
 0:58:08
0:58:08
 0:01:50
0:01:50
 0:01:51
0:01:51
 0:03:47
0:03:47
 0:01:19
0:01:19
 0:01:45
0:01:45
 0:01:15
0:01:15
 0:05:42
0:05:42
 0:01:11
0:01:11
 0:01:29
0:01:29
 0:01:21
0:01:21
 0:01:25
0:01:25
 0:02:15
0:02:15
 0:01:59
0:01:59
 0:00:16
0:00:16
 0:00:59
0:00:59