filmov
tv
Lone Pair vs Bonding Pair Electrons

Показать описание
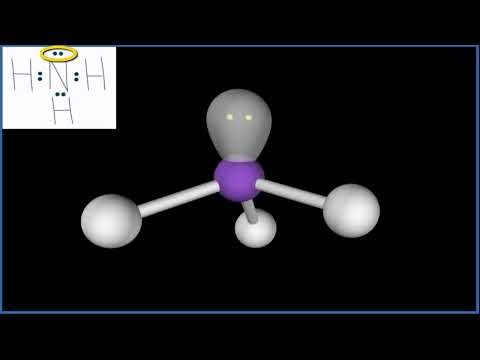
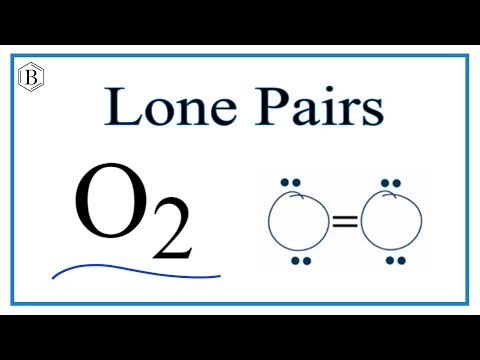
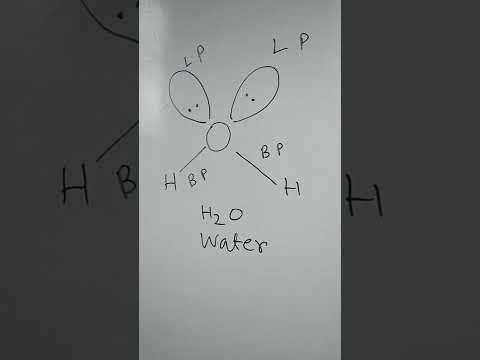
Lone pairs and bonding pairs of electrons refer to the electrons in the valence shell of an atom. Lone pairs are electrons that are not shared in a chemical bond. Bonding pairs, on the other hand, are electrons that are shared in a chemical bond between two atoms.
Lone pairs of electrons are important in a number of ways. One of the key reasons is that they play a crucial role in determining the chemical reactivity and physical properties of a molecule. For example, lone pairs also affect the geometry of a molecule. They occupy space and can cause a molecule to adopt a specific shape. The shape of a molecule can affect its reactivity, stability, and physical properties such as boiling point and melting point.
Lone pairs also play a role in determining the polarity of a molecule. A polar molecule has a positive and negative end, while a nonpolar molecule does not. The presence of lone pairs can make a molecule polar, which can affect its solubility and other properties.
Finally, lone pairs are also important in biological systems, where they play a role in the functioning of enzymes, hormones, and other biomolecules.
Helpful Resources:
Lone pairs of electrons are important in a number of ways. One of the key reasons is that they play a crucial role in determining the chemical reactivity and physical properties of a molecule. For example, lone pairs also affect the geometry of a molecule. They occupy space and can cause a molecule to adopt a specific shape. The shape of a molecule can affect its reactivity, stability, and physical properties such as boiling point and melting point.
Lone pairs also play a role in determining the polarity of a molecule. A polar molecule has a positive and negative end, while a nonpolar molecule does not. The presence of lone pairs can make a molecule polar, which can affect its solubility and other properties.
Finally, lone pairs are also important in biological systems, where they play a role in the functioning of enzymes, hormones, and other biomolecules.
Helpful Resources:
Комментарии
 0:03:47
0:03:47
 0:01:42
0:01:42
 0:01:11
0:01:11
 0:06:49
0:06:49
 0:06:31
0:06:31
 0:10:09
0:10:09
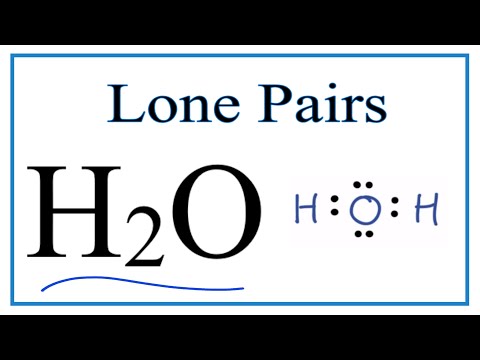 0:01:57
0:01:57
 0:00:57
0:00:57
 2:27:27
2:27:27
 0:01:18
0:01:18
 0:00:19
0:00:19
 0:05:31
0:05:31
 0:00:18
0:00:18
 0:11:38
0:11:38
 0:02:11
0:02:11
 0:01:11
0:01:11
 0:01:17
0:01:17
 0:00:16
0:00:16
 0:01:00
0:01:00
 0:21:56
0:21:56
 0:01:03
0:01:03
 0:01:09
0:01:09
 0:13:10
0:13:10
 0:03:49
0:03:49