filmov
tv
Rutherford Model of atom #atom #rutherfordatomicmodel #chemistry #education

Показать описание
The Rutherford model of the atom, proposed by Ernest Rutherford in 1911, was a significant advancement in atomic theory, providing a more accurate representation of atomic structure than previous models. Here’s a detailed overview of the Rutherford model:
### **Rutherford Model of the Atom**
#### **Historical Context:**
- Prior to Rutherford's model, the **Thomson model** (also known as the "plum pudding model"), proposed by J.J. Thomson in 1904, suggested that the atom was a uniform, positively charged sphere with electrons embedded randomly within it.
#### **Key Experiments:**
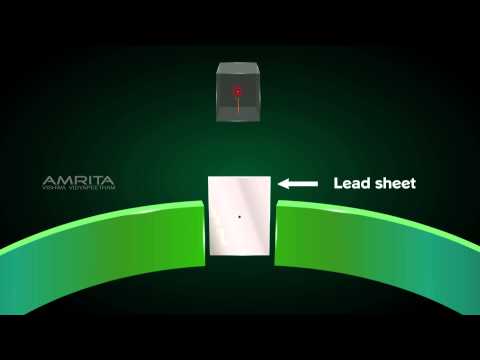
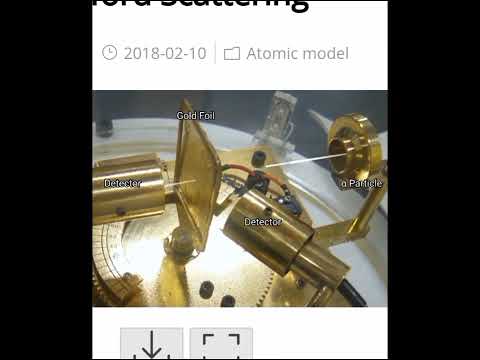
- Rutherford’s model was based on the results of the **gold foil experiment** conducted by Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden in 1909. In this experiment, alpha particles (positively charged particles) were directed at a very thin foil of gold.
#### **Gold Foil Experiment:**
- **Setup:** A beam of alpha particles was directed at a thin foil of gold, surrounded by a fluorescent screen coated with zinc sulfide. The screen was observed through a microscope to detect scattered alpha particles.
- **Observations:**
- Most alpha particles passed straight through the foil without any deflection.
- A small fraction of alpha particles were deflected at large angles.
- Very few alpha particles were deflected back towards the source.
#### **Conclusions and Model:**
1. **Nucleus:**
- Rutherford concluded that most of the atom’s mass and all of its positive charge are concentrated in a small, dense region at the center of the atom, which he called the **nucleus**.
- This nucleus is positively charged, as it repelled the positively charged alpha particles.
2. **Atom Structure:**
- The majority of the atom’s volume is empty space where electrons are located. The electrons orbit the nucleus at a relatively large distance compared to the size of the nucleus.
3. **Electron-Orbit Model:**
- Electrons move around the nucleus in orbits, much like planets orbiting the sun. This was a significant shift from the Thomson model, which did not provide a detailed structure for electron arrangement.
4. **Size of the Nucleus:**
- The nucleus is extremely small compared to the overall size of the atom. While the atom itself has a radius on the order of angstroms (10⁻¹⁰ meters), the nucleus has a radius on the order of femtometers (10⁻¹⁵ meters).
#### **Limitations of the Rutherford Model:**
1. **Electron Stability:**
- According to classical physics, accelerating electrons (which would be moving in circular orbits around the nucleus) should emit radiation and lose energy, causing them to spiral into the nucleus. This did not happen in practice, as atoms are stable.
2. **Electron Energy Levels:**
- The Rutherford model could not explain the discrete line spectra of atoms observed in experiments, where atoms emit or absorb radiation at specific wavelengths.
#### **Legacy and Development:**
- **Bohr Model:** The limitations of Rutherford's model were addressed by Niels Bohr in 1913, who proposed the Bohr model of the atom. Bohr introduced the concept of quantized energy levels for electrons, explaining the stability of electron orbits and the line spectra of atoms.
- **Quantum Mechanics:** The Rutherford model laid the groundwork for the development of quantum mechanics, which provides a more complete and accurate description of atomic structure and electron behavior.
### **Summary:**
The Rutherford model represented a major advancement in atomic theory by introducing the concept of a central nucleus and demonstrating that most of the atom is empty space. Despite its limitations, it provided a critical foundation for later developments in atomic theory, leading to more refined models and the modern understanding of atomic structure.
### **Rutherford Model of the Atom**
#### **Historical Context:**
- Prior to Rutherford's model, the **Thomson model** (also known as the "plum pudding model"), proposed by J.J. Thomson in 1904, suggested that the atom was a uniform, positively charged sphere with electrons embedded randomly within it.
#### **Key Experiments:**
- Rutherford’s model was based on the results of the **gold foil experiment** conducted by Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden in 1909. In this experiment, alpha particles (positively charged particles) were directed at a very thin foil of gold.
#### **Gold Foil Experiment:**
- **Setup:** A beam of alpha particles was directed at a thin foil of gold, surrounded by a fluorescent screen coated with zinc sulfide. The screen was observed through a microscope to detect scattered alpha particles.
- **Observations:**
- Most alpha particles passed straight through the foil without any deflection.
- A small fraction of alpha particles were deflected at large angles.
- Very few alpha particles were deflected back towards the source.
#### **Conclusions and Model:**
1. **Nucleus:**
- Rutherford concluded that most of the atom’s mass and all of its positive charge are concentrated in a small, dense region at the center of the atom, which he called the **nucleus**.
- This nucleus is positively charged, as it repelled the positively charged alpha particles.
2. **Atom Structure:**
- The majority of the atom’s volume is empty space where electrons are located. The electrons orbit the nucleus at a relatively large distance compared to the size of the nucleus.
3. **Electron-Orbit Model:**
- Electrons move around the nucleus in orbits, much like planets orbiting the sun. This was a significant shift from the Thomson model, which did not provide a detailed structure for electron arrangement.
4. **Size of the Nucleus:**
- The nucleus is extremely small compared to the overall size of the atom. While the atom itself has a radius on the order of angstroms (10⁻¹⁰ meters), the nucleus has a radius on the order of femtometers (10⁻¹⁵ meters).
#### **Limitations of the Rutherford Model:**
1. **Electron Stability:**
- According to classical physics, accelerating electrons (which would be moving in circular orbits around the nucleus) should emit radiation and lose energy, causing them to spiral into the nucleus. This did not happen in practice, as atoms are stable.
2. **Electron Energy Levels:**
- The Rutherford model could not explain the discrete line spectra of atoms observed in experiments, where atoms emit or absorb radiation at specific wavelengths.
#### **Legacy and Development:**
- **Bohr Model:** The limitations of Rutherford's model were addressed by Niels Bohr in 1913, who proposed the Bohr model of the atom. Bohr introduced the concept of quantized energy levels for electrons, explaining the stability of electron orbits and the line spectra of atoms.
- **Quantum Mechanics:** The Rutherford model laid the groundwork for the development of quantum mechanics, which provides a more complete and accurate description of atomic structure and electron behavior.
### **Summary:**
The Rutherford model represented a major advancement in atomic theory by introducing the concept of a central nucleus and demonstrating that most of the atom is empty space. Despite its limitations, it provided a critical foundation for later developments in atomic theory, leading to more refined models and the modern understanding of atomic structure.
 0:04:17
0:04:17
 0:04:33
0:04:33
 0:06:43
0:06:43
 0:05:21
0:05:21
 0:05:06
0:05:06
 0:07:04
0:07:04
 0:05:23
0:05:23
 0:03:30
0:03:30
 0:01:00
0:01:00
 0:00:06
0:00:06
 0:04:26
0:04:26
 0:03:28
0:03:28
 0:04:32
0:04:32
 0:06:06
0:06:06
 0:20:03
0:20:03
 0:00:16
0:00:16
 0:00:05
0:00:05
 0:00:05
0:00:05
 0:04:56
0:04:56
 0:06:32
0:06:32
 0:04:27
0:04:27
 0:12:21
0:12:21
 0:00:25
0:00:25
 0:00:48
0:00:48