filmov
tv
Rutherford's Model of An Atom

Показать описание
Rutherford's Model of an Atom
In this module, you will:
⚫ learn about Rutherford's model of an atom.
• Rutherford gave a nuclear model of an atom, which was based on his 'alpha' particle scattering experiment.
• According to the Rutherford's model of an atom:
o All the positive charge and mass of an atom is concentrated at the centre.
o The centre of an atom is called the nucleus.
o The size of the nucleus is very small as compared to the size of the atom.
o The electrons of an atom revolve around the nucleus.
• The major drawback of this model was that it could not explain the stability of the atom.
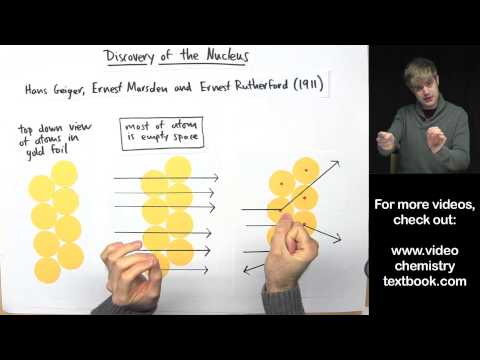
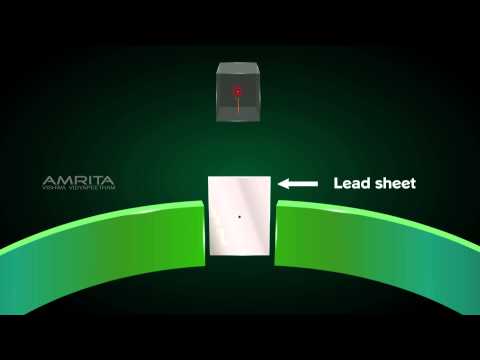
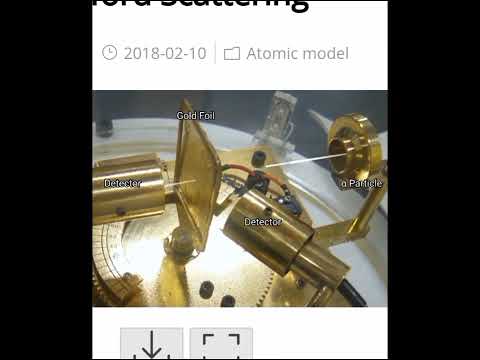
Rutherford performed an alpha particle scattering experiment on gold foil. Alpha particle source was kept in a lead block and a circular screen was placed around the foil. As alpha particles are scattered through the source and struck the zinc sulfide screen, these produce scintillations. Rutherford examined these scintillations in different portions on the screen and observed that most of the alpha particles penetrated through the foil showed no deflection. Rutherford concluded that most of the space in an atom is empty and at its center, there is a positively charged sphere called nucleus around which electrons are revolving in the atom.
AASOKA provides 2D/3D educational videos based on the CBSE curriculum that will help you understand difficult concepts and complicated experiments in a simple, easy-to-understand way. You can check all available videos of this grade and to get more clarity, or for progression of difficulty in different topics of this subject, you can check out the given playlists available on our channel:
To stay up-to-date with exciting updates about our videos and other content, connect with us on:
Please encourage us by liking our videos. Leave a comment about what we did well and how we can improve. Don't forget to subscribe to our channel to watch more educational videos!
Tags:
Rutherford's model
model of an atom
rutherford atomic model
rutherford model experiment
Rutherford's Model of An Atom diagram
#science #rutherfordsmodel #atom #jee #neet
In this module, you will:
⚫ learn about Rutherford's model of an atom.
• Rutherford gave a nuclear model of an atom, which was based on his 'alpha' particle scattering experiment.
• According to the Rutherford's model of an atom:
o All the positive charge and mass of an atom is concentrated at the centre.
o The centre of an atom is called the nucleus.
o The size of the nucleus is very small as compared to the size of the atom.
o The electrons of an atom revolve around the nucleus.
• The major drawback of this model was that it could not explain the stability of the atom.
Rutherford performed an alpha particle scattering experiment on gold foil. Alpha particle source was kept in a lead block and a circular screen was placed around the foil. As alpha particles are scattered through the source and struck the zinc sulfide screen, these produce scintillations. Rutherford examined these scintillations in different portions on the screen and observed that most of the alpha particles penetrated through the foil showed no deflection. Rutherford concluded that most of the space in an atom is empty and at its center, there is a positively charged sphere called nucleus around which electrons are revolving in the atom.
AASOKA provides 2D/3D educational videos based on the CBSE curriculum that will help you understand difficult concepts and complicated experiments in a simple, easy-to-understand way. You can check all available videos of this grade and to get more clarity, or for progression of difficulty in different topics of this subject, you can check out the given playlists available on our channel:
To stay up-to-date with exciting updates about our videos and other content, connect with us on:
Please encourage us by liking our videos. Leave a comment about what we did well and how we can improve. Don't forget to subscribe to our channel to watch more educational videos!
Tags:
Rutherford's model
model of an atom
rutherford atomic model
rutherford model experiment
Rutherford's Model of An Atom diagram
#science #rutherfordsmodel #atom #jee #neet
Комментарии
 0:05:21
0:05:21
 0:04:17
0:04:17
 0:05:06
0:05:06
 0:00:58
0:00:58
 0:04:33
0:04:33
 0:06:43
0:06:43
 0:00:16
0:00:16
 0:07:04
0:07:04
 0:15:59
0:15:59
 0:03:30
0:03:30
 0:00:22
0:00:22
 0:03:25
0:03:25
 0:00:06
0:00:06
 0:06:32
0:06:32
 0:03:43
0:03:43
 0:00:59
0:00:59
 0:00:25
0:00:25
 0:00:56
0:00:56
 0:06:22
0:06:22
 0:00:29
0:00:29
 0:00:05
0:00:05
 0:00:16
0:00:16
 0:06:06
0:06:06
 0:18:04
0:18:04