filmov
tv
Physics 32.5 Statistical Thermodynamics (26 of 39) What is Entropy of 1 mol of Gas Distributed?

Показать описание
To donate:
Now that we know how to calculate Sterling’s approximation, we will use it to calculate the entropy of 1 mole of gas molecules evenly distributed.
Next video in this series can be seen at:
Physics 32.5 Statistical Thermodynamics (26 of 39) What is Entropy of 1 mol of Gas Distributed?
Physics 32.5 Statistical Thermodynamics (5 of 39) The Average Occupation Number
Physics 32.5 Statistical Thermodynamics (4 of 39) Understanding Statistical Thermodynamics 2
delhi civil defence m aise ho rhe h kaam #shorts #delhi #indian
Most💯 Important Step Before any Procedure 🔥
11 years later ❤️ @shrads
NEWYES Calculator VS Casio calculator
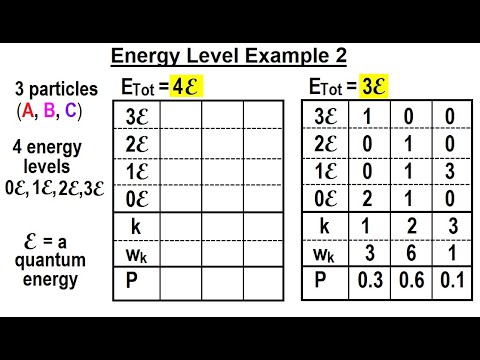
Physics 32.5 Statistical Thermodynamics (33 of 39) Energy Level Example 2
Physics 32.5 Statistical Thermodynamics (27 of 39) Entropy Change for Moving N Molecules
Physics 32.5 Statistical Thermodynamics (24 of 39) N Molecules in a Box: Divided in N Equal Sections
Physics 32.5 Statistical Thermodynamics (38 of 39) Find the Quantum Number of Volume L^3 of He Ex1
😁 Playing 🐍Snake🐍 game on calculator 😜 [official video] #shorts #viral #casio
Physics 32.5 Statistical Thermodynamics (31 of 39) General Counting Method for w
xavier memes #memes
Salsa Night in IIT Bombay #shorts #salsa #dance #iit #iitbombay #motivation #trending #viral #jee
TRICKS you can do in SCIENTIFIC CALCULATORS🔥#viral #shorts
#pov : my gcse results vs what i predicted #gcse #gcseresults #gcse2022 #results #shortsvideo
Physics 32.5 Statistical Thermodynamics (39 of 39) Find the Quantum Number of Volume L^3 of He Met 2
Physics 32.5 Statistical Thermodynamics (20 of 39) 6 Molecules in a Box: Divided in 3 Equal Sections
Growing up Pentecostal... #short
26. Ideal Paramagnet II -- Course in Thermal and Statistical Physics
Bro’s hacking life 😭🤣
Emporium mall me Is larki ki bygarti deko
Jaldi Wahan Se Hato! IIT Delhi version! #iit #iitjee #iitdelhi
Комментарии
 0:04:57
0:04:57
 0:03:20
0:03:20
 0:05:09
0:05:09
 0:00:13
0:00:13
 0:00:16
0:00:16
 0:00:11
0:00:11
 0:00:14
0:00:14
 0:07:02
0:07:02
 0:04:55
0:04:55
 0:04:00
0:04:00
 0:04:12
0:04:12
 0:00:47
0:00:47
 0:07:52
0:07:52
 0:00:06
0:00:06
 0:00:14
0:00:14
 0:00:25
0:00:25
 0:00:16
0:00:16
 0:02:48
0:02:48
 0:05:43
0:05:43
 0:00:15
0:00:15
 0:39:46
0:39:46
 0:00:20
0:00:20
 0:00:18
0:00:18
 0:00:17
0:00:17