filmov
tv
Medical Device Software Development Short Course

Показать описание
This is a short course on medical device software development. The goal is to give you a basic understanding of some key concepts relating to the development of medical device software.
Don't miss checking out the online course Introduction to SaMD and IEC 82304-1:
Starting October 2023, the templates mentioned in the video are no longer available for free on Medical Device HQ. However, more comprehensive paid versions are now accessible via this link:
We offer premium templates for free to our newsletter subscribers. Instant access to some templates is granted upon subscription. Others will be distributed periodically as part of our newsletter email campaigns.
Chapters:
00:00 Introduction
00:24 About the instructor
01:10 Who is this course for?
01:18 Learning goals
01:53 Introduction to the IEC 62304 standard
02:55 Key elements of the IEC 62304 standard
04:09 The scope of the IEC 62304 standard
07:19 Scrum (Agile) vs IEC 62304
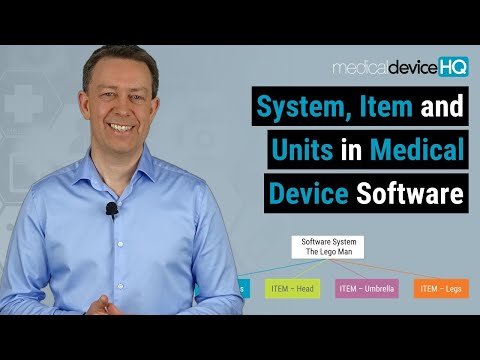
09:14 Medical software safety classification
10:20 Medical software development planning
11:08 Documenting software development planning
13:34 What is legacy software?
14:02 How to use the legacy clause
16:41 Configuration management in software development
17:33 Version control systems
20:01 Understanding probability of occurrence of harm
22:22 Additional help and resources
Don't miss checking out the online course Introduction to SaMD and IEC 82304-1:
Starting October 2023, the templates mentioned in the video are no longer available for free on Medical Device HQ. However, more comprehensive paid versions are now accessible via this link:
We offer premium templates for free to our newsletter subscribers. Instant access to some templates is granted upon subscription. Others will be distributed periodically as part of our newsletter email campaigns.
Chapters:
00:00 Introduction
00:24 About the instructor
01:10 Who is this course for?
01:18 Learning goals
01:53 Introduction to the IEC 62304 standard
02:55 Key elements of the IEC 62304 standard
04:09 The scope of the IEC 62304 standard
07:19 Scrum (Agile) vs IEC 62304
09:14 Medical software safety classification
10:20 Medical software development planning
11:08 Documenting software development planning
13:34 What is legacy software?
14:02 How to use the legacy clause
16:41 Configuration management in software development
17:33 Version control systems
20:01 Understanding probability of occurrence of harm
22:22 Additional help and resources
Комментарии
 0:23:06
0:23:06
 0:28:40
0:28:40
 0:27:12
0:27:12
 0:07:07
0:07:07
 0:05:33
0:05:33
 0:11:35
0:11:35
 0:09:31
0:09:31
 0:12:03
0:12:03
 3:52:55
3:52:55
 0:03:05
0:03:05
 0:25:20
0:25:20
 0:12:34
0:12:34
 1:11:09
1:11:09
 0:12:48
0:12:48
 0:02:15
0:02:15
 0:12:24
0:12:24
 0:14:56
0:14:56
 0:01:00
0:01:00
 0:11:26
0:11:26
 0:06:37
0:06:37
 0:26:07
0:26:07
 0:38:45
0:38:45
 0:14:55
0:14:55
 0:10:44
0:10:44