filmov
tv
Calculate the wavelength of the photon emitted when the hydrogen atom transition from n=5 to n=3.

Показать описание
Calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3.
Speed of Light, Frequency, and Wavelength Calculations - Chemistry Practice Problems
How To: Find Wavelength / Frequency (EASY EQUATION w/ problems)
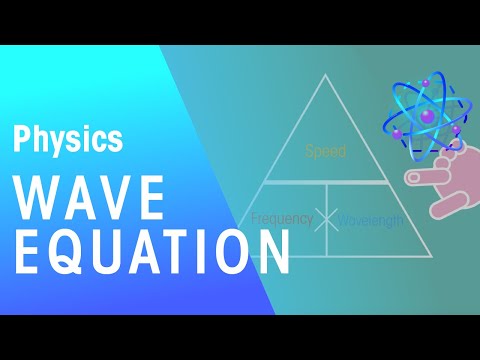
Using the Wave Equation (Wavelength, Speed and Frequency)
Amplitude and Wavelength of a Wave - WORKED EXAMPLE - GCSE Physics
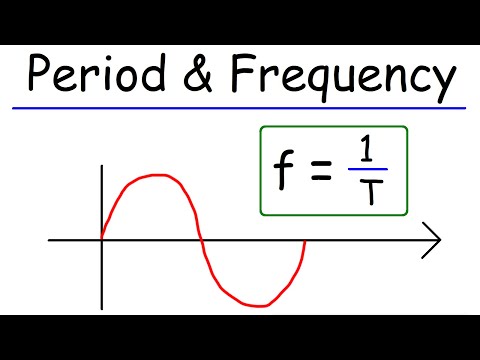
Period, Frequency, Amplitude, & Wavelength - Waves
Calculating wavelength of a wave
Wave Equation | Waves | Physics | FuseSchool
Calculate the wavelength of the photon emitted when the hydrogen atom transition from n=5 to n=3.
9702/M/J/21/2024 | Physics P2 | A Level | CAIE
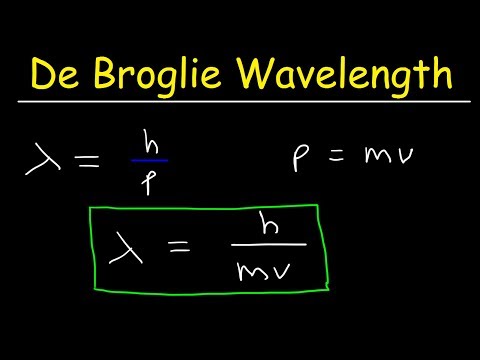
De Broglie Wavelength Problems In Chemistry
ALEKS: Calculating the wavelength of a spectral line from an energy diagram
How to calculate the Wavelength
How to Find the Wavelength, Frequency, Energy and Photons | Study Chemistry With Us
How To Calculate The Energy of a Photon Given Frequency & Wavelength in nm Chemistry
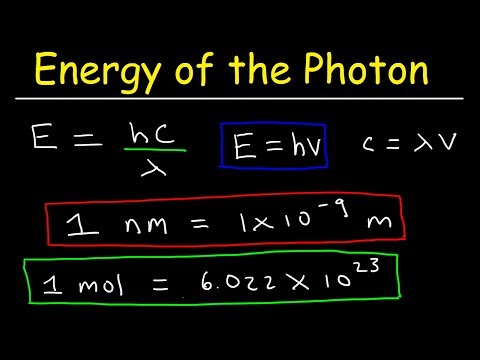
Energy from Wavelength: Electromagnetic Radiation Calculation
ALEKS: Calculating the wavelength of a line in the spectrum of hydrogen
How to Calculate Wavelength of Light | Chemistry Homework in 3 MINUTES
How to Calculate the Wavelength of a Wave When Wave Speed and Frequency are Known
How to Calculate the Wavelength of the First Three Lines in the Balmer Ser... : Chemistry & Phys...
Calculating the Wavelength of a Laser
Calculating the wavelength for the Balmer Series
Calculate (a) wavenumber and (b) frequency of yellow radiation having wavelength 5800 Å.
How to calculate wavelength and frequency of radio waves?
Calculating the Wavelength of Microwaves - Mr Pauller
Комментарии
 0:11:36
0:11:36
 0:04:05
0:04:05
 0:03:13
0:03:13
 0:02:09
0:02:09
 0:12:43
0:12:43
 0:04:39
0:04:39
 0:03:24
0:03:24
 0:02:54
0:02:54
 1:03:57
1:03:57
 0:11:21
0:11:21
 0:07:46
0:07:46
 0:00:36
0:00:36
 0:36:02
0:36:02
 0:11:06
0:11:06
 0:04:43
0:04:43
 0:07:25
0:07:25
 0:01:41
0:01:41
 0:03:39
0:03:39
 0:04:08
0:04:08
 0:01:36
0:01:36
 0:04:56
0:04:56
 0:03:35
0:03:35
 0:06:29
0:06:29
 0:02:37
0:02:37