filmov
tv
Proton NMR Spectrum Example

Показать описание
How To Draw The Proton NMR Spectrum of an Organic Molecule
Proton NMR Spectrum Example
Proton NMR Spectroscopy - How To Draw The Structure Given The Spectrum
Proton NMR practice 1 | Spectroscopy | Organic chemistry | Khan Academy
Proton NMR Spectroscopy: What You Need to Know // HSC Chemistry
NMR Analysis - Assigning a Spectrum and Predicting a Structure (Harder Version)
NMR Spectroscopy for Visual Learners
Proton NMR example 1
H-NMR Predicting Molecular Structure Using Formula + Graph
Integration of H NMR Signals - Spectroscopy - Organic Chemistry
NMR Spectroscopy
How To Determine The Number of Signals In a H NMR Spectrum
1H NMR - Spectra Interpretation Part I Examples
How to read Proton NMR spectroscopy graphs + Practice (EXAMPLE 1)
H-NMR Example Matching The Molecule To The Graph
Proton NMR - How To Analyze The Peaks Of H-NMR Spectroscopy
NMR Spectroscopy Interpretation (Example)
Applying Proton NMR Spectroscopy
Analyzing Proton NMR Spectra
Proton NMR Spectroscopy (A-level IB Chemistry)
NMR Spectroscopy Practice Problems - Solving NMR Step by Step
Organic Chemistry - How to Solve NMR Problems
How to Read Proton NMR Spectroscopy graphs + Practice (Example 2)
NMR Example Questions from www.ChemistryTuition.Net
Комментарии
 0:12:06
0:12:06
 0:05:41
0:05:41
 0:14:12
0:14:12
 0:11:36
0:11:36
 0:07:38
0:07:38
 0:11:19
0:11:19
 0:23:55
0:23:55
 0:02:12
0:02:12
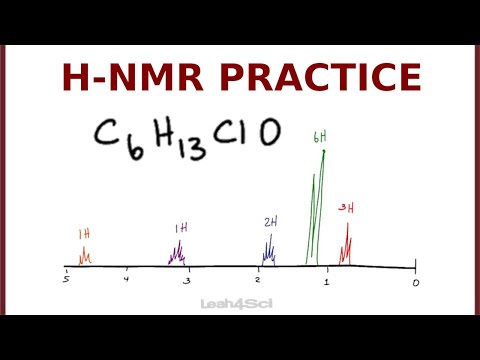 0:11:02
0:11:02
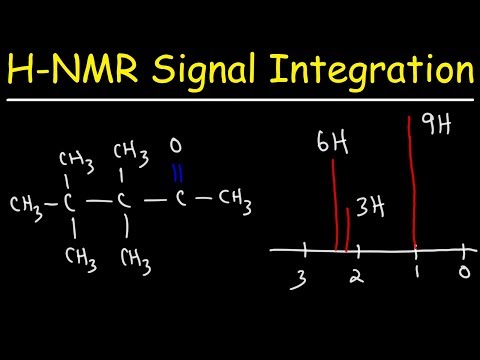 0:05:29
0:05:29
 0:14:36
0:14:36
 0:20:26
0:20:26
 0:10:19
0:10:19
 0:07:40
0:07:40
 0:06:43
0:06:43
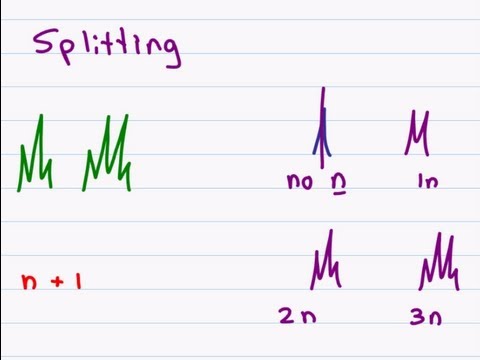 0:11:31
0:11:31
 0:02:45
0:02:45
 0:12:25
0:12:25
 0:18:19
0:18:19
 0:22:20
0:22:20
 0:13:44
0:13:44
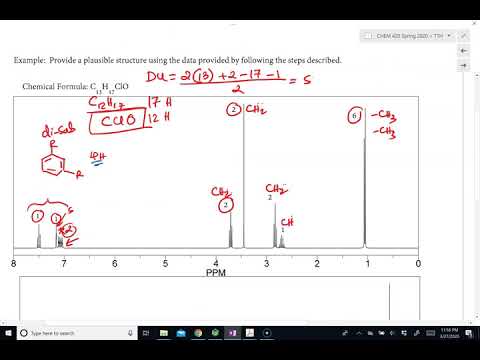 0:31:38
0:31:38
 0:08:15
0:08:15
 0:09:41
0:09:41