filmov
tv
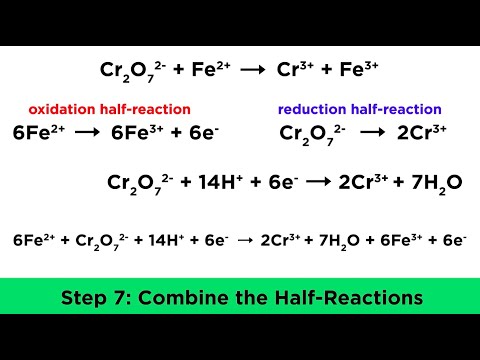
Balance the Redox Reaction for Cr2O7 2- + Fe2+ → Cr3+ + Fe3+

Показать описание
In this video I’ll walk you through the process for successfully balancing this reduction-oxidation reaction. We'll use the ion-electron method, also called the half-reaction method, to balance this reduction-oxidation reaction.
----Helpful Resources-----
----- Five Steps to Balance Redox Reactions ----
1) Write the oxidation numbers for each element.
2) Write the half reactions for the species of interest.
3) Balance each half-reactions for:
- atoms of interest.
- Oxygen (O) atoms by adding H2O.
- Hydrogen (H) atoms by adding H+ ions.
- electrons (charge) by adding electrons.
4) Balance the overall equation for electrons (charge).
5) Add half reactions and simplify.
Redox reactions, like Cr2O7 2- + Fe2+ Cr3+ + Fe3+ , are one of them most challenging topics in chemistry. They require careful attention to detail and a strong conceptual understanding of oxidation and reduction. To be successful:
- Check to make sure you have the equation written correctly.
- Get the oxidation numbers correct first!
- Take your time. Simple mistakes are easy to make.
- Think about what is happening and if it makes sense.
- Check your work. Are the number of atoms on each side equal? Does charge balance?
Finally, becoming proficient at balancing redox reactions requires lots and lots of practice. This is especially true because of the many steps and things to keep track of.
Комментарии
 0:07:31
0:07:31
 0:16:00
0:16:00
 0:03:35
0:03:35
 0:18:00
0:18:00
 0:12:39
0:12:39
 0:15:00
0:15:00
 0:04:33
0:04:33
 0:32:49
0:32:49
 0:33:29
0:33:29
 0:08:22
0:08:22
 0:03:56
0:03:56
 0:12:02
0:12:02
 0:03:46
0:03:46
 0:14:03
0:14:03
 0:05:32
0:05:32
 0:15:55
0:15:55
 0:04:17
0:04:17
 0:04:41
0:04:41
 0:05:10
0:05:10
 0:06:55
0:06:55
 0:05:05
0:05:05
 0:16:05
0:16:05
 0:01:00
0:01:00
 0:04:26
0:04:26