filmov
tv
Separating Liquids by Distillation
Показать описание
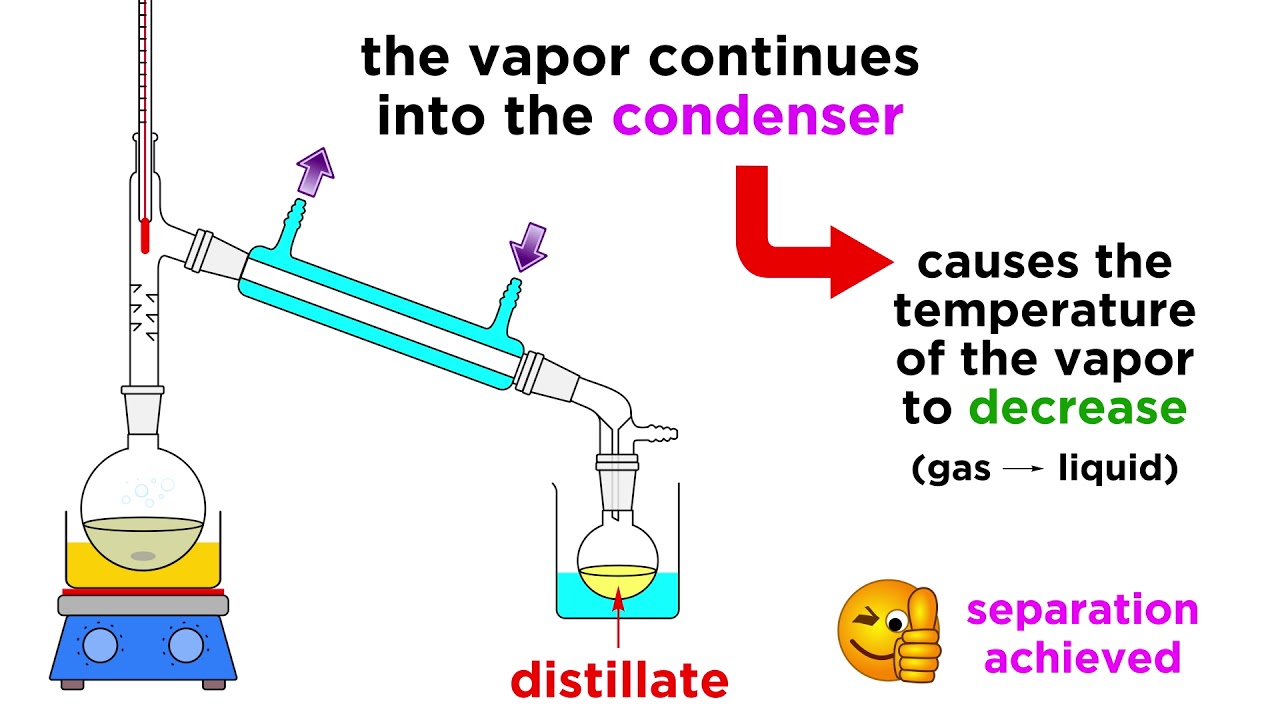
We've got extraction and chromatography down, so let's learn one more separation technique. This one is pretty simple, it separates mixtures of liquids by differences in boiling point, and it's called distillation. If two liquids have very different boiling points, we should be able to heat the mixture up until one boils and the other doesn't, and we can collect the vapor somewhere else, and that's all there is to it. It's quite simple, in fact, but there are some important tips regarding the apparatus, so let's take a look!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Separating Liquids by Distillation
GCSE Chemistry - Fractional Distillation and Simple Distillation #50
Separating Solutions – Distillation
Separation of Mixtures: Distillation
Simple Distillation
Simple Distillation: Separation Technique of Miscible Liquids
Distillation process of acetone and water mixture #chemistry #science #experiment #virashorts #viral
Separation of Liquids by Distillation | GCSE Chemistry
UTME Jamb Chemistry Tutorial 2025, Separation of Mixtures and Purification of Chemical Substances
Different boiling points and distillation| Separation Methods | Chemistry
Simple Distillation | #aumsum #kids #science #education #children
GCSE Chemistry - Filtration, Evaporation & Crystallisation #6
Distillation and phase equilibria
Simple Distillation
Simple Distillation | #science #analyticalchemistry #edexmy
To Separate a Saltwater Mixture by Distillation
⚗️Distillation🧪| How to Separate Two Miscible (also Immiscible) Liquids | Class 9 Science Chapter 2...
Introduction to Fractional distillation | Distillation procedure| Home Revise | Chemistry Experiment
Separating Mixtures: Distillation | 9-1 GCSE Science Chemistry | OCR, AQA, Edexcel
How to separate miscible liquids| What is Fractional Distillation| Separation Methods
Simple Distillation #simple #distillation
Separation of Liquid - Liquid Mixtures - Fractional Distillation
Sec 1 science revision - separation techniques. Simple distillation - miscible liquids!
Distillation Process
Комментарии
 0:05:57
0:05:57
 0:05:35
0:05:35
 0:03:38
0:03:38
 0:01:54
0:01:54
 0:09:35
0:09:35
 0:03:38
0:03:38
 0:00:25
0:00:25
 0:03:46
0:03:46
 0:24:46
0:24:46
 0:02:09
0:02:09
 0:06:16
0:06:16
 0:04:17
0:04:17
 0:03:51
0:03:51
 0:00:11
0:00:11
 0:00:20
0:00:20
 0:04:10
0:04:10
 0:00:59
0:00:59
 0:01:45
0:01:45
 0:04:59
0:04:59
 0:05:50
0:05:50
 0:00:05
0:00:05
 0:01:58
0:01:58
 0:00:16
0:00:16
 0:00:15
0:00:15