filmov
tv
6. Hydrogen Atom Wavefunctions (Orbitals)

Показать описание
MIT 5.111 Principles of Chemical Science, Fall 2014
Instructor: Catherine Drennan
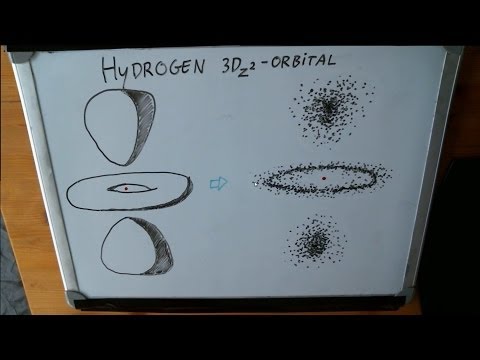
Where is that electron anyway? In this lecture, the probability of finding an electron at a particular distance from the nucleus is discussed. The concept of wavefunctions (orbitals) is introduced, and applications of electron spin are described. In particular, chemist Ben Ofori-Okai introduces us to the wonders of magnetic resonance imaging, also known as MRIs.
License: Creative Commons BY-NC-SA
Instructor: Catherine Drennan
Where is that electron anyway? In this lecture, the probability of finding an electron at a particular distance from the nucleus is discussed. The concept of wavefunctions (orbitals) is introduced, and applications of electron spin are described. In particular, chemist Ben Ofori-Okai introduces us to the wonders of magnetic resonance imaging, also known as MRIs.
License: Creative Commons BY-NC-SA
6. Hydrogen Atom Wavefunctions (Orbitals)
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Transitions in hydrogen wavefunctions
The Hydrogen Atom, Part 1 of 3: Intro to Quantum Physics
How orbitals arise from atomic wave functions (U2-08-07)
Hydrogen atom: 2p₀ orbital
Quantum Mechanics: Schrödinger's discovery of the shape of atoms
Quantum Chemistry 7.3 - Hydrogen Atom Radial Wavefunctions
Hydrogen Atom and the Periodic Table Part 1: Quantum Mechanics Made Easy
The hydrogen atom. Wavefunctions
Atomic Orbitals, Visualized Dynamically
Wave Functions of 2p Orbitals of Hydrogen Atom
Chapter 6: Introduction to the Hydrogen Atom | CHM 309 | 061
Schrodinger equation solutions to the hydrogen atom
6. Electron Shell Model, Quantum Numbers, and PES (Intro to Solid-State Chemistry)
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
Quantum Chemistry 7.5 - Hydrogen Atomic Orbital Nodes
H Atom Wavefunction Characteristics (Summary)
Latest Image of An Atom! 🔬
Hydrogen Atom Wave Function - Find the type of Orbitals
Hydrogen atom quantum mechanics|Wave function|How to find n l m quantum numbers|Spherical harmonics
6 36 Schrodinger's Equation and Atomic Orbitals
Quantum Chemistry 7.4 - Hydrogen Atom Total Wavefunctions (Old Version)
Quantum Chemistry 7.5 - Hydrogen Atomic Orbital Nodes (Old Version)
Комментарии
 1:00:39
1:00:39
 0:08:42
0:08:42
 0:00:41
0:00:41
 0:18:35
0:18:35
 0:02:07
0:02:07
 0:00:36
0:00:36
 0:07:18
0:07:18
 0:09:28
0:09:28
 0:20:48
0:20:48
 0:14:40
0:14:40
 0:08:39
0:08:39
 0:10:29
0:10:29
 0:03:58
0:03:58
 0:17:27
0:17:27
 0:48:35
0:48:35
 0:21:44
0:21:44
 0:07:10
0:07:10
 0:07:16
0:07:16
 0:00:24
0:00:24
 0:11:36
0:11:36
 0:40:54
0:40:54
 0:03:59
0:03:59
 0:11:20
0:11:20
 0:10:18
0:10:18