filmov
tv
Mean Free Path

Показать описание
In a gas, molecules undergo collisions with one another. How far do they travel, on average, between collisions?
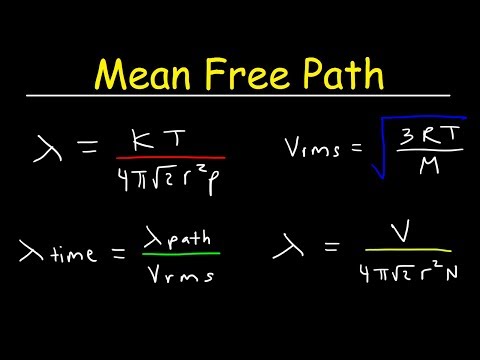
Mean Free Path, Mean Free Time, & Root Mean Square Velocity Formula Chemistry & Physics Pr...
The mean free path explained
Mean Free Path
Mean Free Path
Mean Free Path
Mean Free Path
Class 11th – Mean Free Path | Kinetic Theory of Gases | Tutorials Point
Mean free path and collision frequency (derivation)
Mean free path | Derivation of mean free path | Collision Probability | Lecture 6
Kinetic energy of a molecule & the Mean free path #7
Mean Free Path Example
Physics - E&M: Ch 40.1 Current & Resistance Understood (10 of 17) What is the Mean Free Path...
Mean Free Path with PYQs #jee #neet Vikrant Kirar
Mean Free Path | YOLO JEE Advance Physics with Vikrant Kirar
Mean Free Path II Dr Rizwana
Mean Free Path|Mean Time|CONCEPTUAL PHYSICS
mean free path || mean free path of gas molecules || bsc 2nd year || bindas physics
Mean free path
Mean Free Path NEET PYQs | Vikrant Kirar
mean free path (hindi)
MEAN FREE PATH || DERIVATION OF MEAN FREE PATH || KINETIC THEORY OF GASES || WITH EXAM NOTES ||
Mean Free Path
Fact or Fib? Test your understanding of the electron mean free path
Collision Frequency, Mean free time (Relaxation time) & Mean Free Path | JEE Main Questions
Комментарии
 0:12:10
0:12:10
 0:02:44
0:02:44
 0:07:38
0:07:38
 0:17:28
0:17:28
 0:03:39
0:03:39
 0:07:46
0:07:46
 0:03:22
0:03:22
 0:18:39
0:18:39
 0:12:19
0:12:19
 0:11:57
0:11:57
 0:04:16
0:04:16
 0:03:44
0:03:44
 0:06:18
0:06:18
 0:20:03
0:20:03
 0:08:04
0:08:04
 0:04:00
0:04:00
 0:05:20
0:05:20
 0:05:54
0:05:54
 0:08:04
0:08:04
 0:12:31
0:12:31
 0:24:41
0:24:41
 0:11:19
0:11:19
 0:01:54
0:01:54
 0:34:21
0:34:21